Background:
Allogeneic hematopoietic stem cell transplant (HSCT) is the only potential curative modality for high risk acute myelogenous leukemia (AML). However, relapse disease remains to be the main reason of failure of allogeneic HSCT. Salvage therapy for relapse AML after allogeneic HSCT is limited and rarely successful. Prognosis is poor with a 2-year survival rate of less than 20%. As majority of relapses occur within first 6 months after transplant, effective preventive intervention given early post allogeneic HSCT may be crucial. An ideal drug for pharmacologic maintenance is difficult to achieve as it should have activity against the disease without excessive myelosuppression or eradication of donor cells, and has minimal or low risk of exacerbation of graft-versus-host disease or additional toxicity.
Azacitidine and decitabine are hypomethylating agents (HMAs), which work epigenetically to modulate various genes, including tumor suppressor genes. They have been shown to have benefits in improvement of event free survival and overall survival in relapse AML. Similar benefits have also been reported when low dose azacitadine was given after allogeneic stem cell transplant as a maintenance therapy.
Method:
This is a single institutional study carried out at the Bone Marrow Transplant Program at the University of Oklahoma Health Science Center. It was started as a case control study in June 2014 when post-transplant azacitadine protocol was initiated, and continues on as a prospective study. Subjects over 18 years old with high risk AML based on cytogenetic and molecular mutation undergoing allogeneic HSCT were enrolled from September 2013 to July 2018. The study is to compare outcome in patients received post-transplant low dose azacitidine at 32mg/m2 on days 1-5 every 28 days for 4 cycles initiating between 6-8 weeks post-transplant versus post-transplant observation. Multivariable cox proportional hazards model was derived to adjust for covariates that differ between the two treatment arms and was used to analyze time to relapse and overall survival in both arms; then our results were graphed using Kaplan Meier Plot.
Results:
This study included 49 patients undergoing allogeneic HSCT for high risk AML. They were divided into 2 groups with 31 patients received post-transplant azacitidine, and 18 patients did not (prior to initiation of post-transplant azacitadine protocol, control).
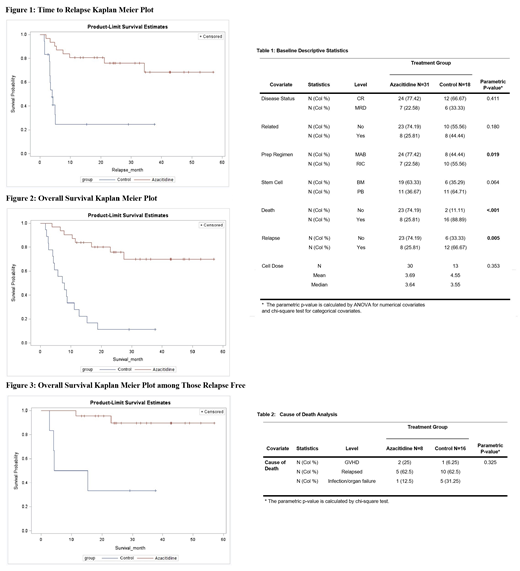
Our Analysis concluded a greater proportion of deaths among the control group (88.89%) compared to the azacitidine group (25.81%). Majority of deaths in the control group was due to relapse (12 of 16 deaths). Relapse rate was higher in the control group (66.67%) as compared to azacitidine group (25.81%) with statistically significant improvement in time to relapse in the azacitidine group (not reach (NR)) versus 4.1 months in the control arm (log-rank p-value <0.0001) which was described in Figure (1).
Median Overall survival was 7.6 months in control arm versus 27.4 months in the 8 patients who relapsed in azacitidine arm (log-rank p-value<0.0001). (Figure 2)
Among the 29 subjects from both arms who were relapse-free, overall survival differed between the two treatment groups (log-rank p-value 0.003). Median survival time among those in the control group was 9.8 months. For the azacitidine arm, only 2 events occurred among the 23 relapse-free subjects while the remaining 21 subjects were censored (Figure 3).
Among other transplant-related factors, disease status was not differed among the two groups (p=0.411), but conditioning regimen was (p=0.019) with a greater proportion of reduced intensity conditioning (RIC) in the control group (55.56%) than azacitadine group (22.58) (Table 1). After adjusting for conditioning regimen, hazard of relapse was 5.26 (1.99,13.93) times greater (p value<0.001) and death was 5.41 (1.99,13.93) times greater (p value<0.001) in the control group than azacitadine group.
In our analysis, cause of death was not statistically significantly different among the two arms (p-value 0.325). Use of azacitidine post-HSCT did not increase the risk of GVHD or infections (table 2).
Implications:
Low dose azacitidine maintenance therapy after allogeneic stem cell transplant in high risk acute myelogenous leukemia decreases relapse rate, time to relapse and improved overall survival without increasing adverse events and no increased risk of graft-versus-host disease or infections.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal