Introduction
DLIs represent a major therapeutic approach for relapse and mixed chimerism (MC) after allogeneic hematopoietic cell transplant (AlloHCT). DLI studies have identified several variables with impact on response and GvHD. Despite some studies having explored the role of T-cells and other cell subsets, such as mononuclear cells (MNCs), comprehensive data regarding the cellular composition of DLI and its role in GvHD remains incomplete, as the development of GvHD post DLI is often unpredictable. Herein we analyzed the cellular composition of DLI from fully human leukocyte antigen (HLA) identical sibling (HLA Id Sib) donors and its impact on the development of GvHD in patients who underwent AlloHCT for hematological malignancy, and its impact on the development of GvHD.
Methods
Inclusion criteria were as follows: 1) Patients ≥ 18 years-old, 2) AlloHCT, 3) HLA Id Sib donor; 4) treatment with DLI; and 5) signed informed consent of patient and donor. Exclusion criteria were: 1) unrelated or mismatched related donors, 2) HCT2 prior to DLI, or 3) GvHD at DLI. For the purpose of avoiding bias, only the cell composition of the first DLI (DLI1) was analyzed. The following cell subsets of the DLI were studied: CD3+, CD4+, CD8+, CD16+CD56+CD3+ (NKT-cell), CD3+CD45RA+CCR7+ (TN), CD3+CD45RA+CCR7+CD31+ (TRTE), CD3+CD45RA+CD95+CD27+ (TSCM), CD3+CD45RA-CCR7+ (TCM), CD3+CD45RA-CCR7- (TEM), CD3+CD45RA+CCR7- (TTE), CD3+CD4+CD25brightCD127dim (TREG), CD3+CD4+CD25brightCD45RA+CD127dim (naïve TREG). The TN, TCM, TEM and TEM compartment was analyzed for both CD4+ and CD8+. We also analyzed the MNCs, CD19+ (B-cell), CD27+CD19+ (mature B-cell), CD16+CD56+CD27- (natural killer (NK+) cell) and CD16+CD56+CD27+ (CD27+NK+cell).
Results
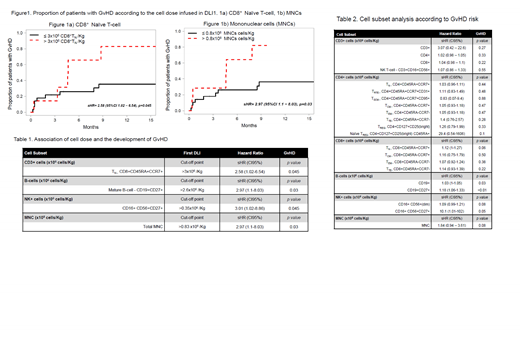
Fifty-six DLIs were infused in 36 patients; the median number of DLI was 1 per patient (range, 1-3). Diagnoses were as follows: 13 AML/MDS, 6 HL, 5 MPN, 4 NHL, 4 CLL, 3 MM and 1 B-ALL. For the study, a landmark analysis was performed from the DLI date. The median follow up from DLI was 282 days (range, 9-5,560 days). Overall response rate in relapsed patients was 29% (9 of 31 patients; 6 CR and 3 PR, most responses being observed after DLI1. Further, five patients had DLI for MC and full donor chimerism was achieved in all patients. Thirteen patients (36%) developed GvHD post DLI. Two patients had GvHD before DLI, but there was no case of GvHD at DLI. The median time interval form DLI to GvHD was 76 days (range, 7-261). As per clinical presentation, 10 patients (27%) had acute GvHD, whereas eight patients (22%) had chronic GvHD. The 6-month and 1-year cumulative incidence (CI) of GvHD was 33% and 46%, respectively. When the risk of GvHD was analyzed according to DLI cell subsets, we observed that a DLI1 containing >3x106 CD8+TN correlated with an increased incidence of GvHD (Figure 1a). Also, a DLI1 with >0.8x108 MNCs/Kg (Figure 1b), >2.6x106 mature B-cell/Kg, or >0.35x106 CD27+NK+cells/Kg were linked to the development of GvHD (Table 1). Noteworthy, CD3+, TN (both CD4+ and CD8+ combined) or CD4+TN had no impact on the development of GvHD; and a high proportion of TREG was not protective for the development of GvHD (Table 2). Finally, there was no statistically significant association between any clinical variable and GvHD.
Conclusion
In conclusion, in this cohort of AlloHCT patients from HLA Id Sib donors, a DLI1 containing a high proportion of CD8+TN, but not CD4+TN, increased the probability of developing GvHD. Further, a DLI1 containing a high dose of MNCs, CD27+NK+cells and mature B-cell also associated with GvHD. These data provide novel insight for the understanding of GvHD post DLI. A DLI1 containing a lower dose of CD8+TN could reduce the risk of GvHD, but this asset warrants further validation in larger cohorts, and within a controlled randomized trial setting.
Bosch:F. Hoffmann-La Roche Ltd/Genentech, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Kyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Honoraria, Research Funding; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal