
Introduction:
Apixaban 2.5mg twice daily has been shown to significantly reduce the risk of venous thromboembolism (VTE) compared to placebo for the primary thromboprophylaxis of VTE in ambulatory cancer patients initiating chemotherapy, who are at intermediate-high risk of VTE, with a Khorana score of ≥2 (hazard ratio, 0.41; 95% confidence interval, 0.26 to 0.65; P<0.001). In this study, we estimated whether the health benefit gained from apixaban justified its costs.
Method:
We conducted a cost-utility analysis of apixaban (2.5mg twice daily) compared to usual care, whereby no apixaban is prescribed, from the perspective of Canada's healthcare system. Our target population was ambulatory cancer patients starting chemotherapy with an intermediate-high risk of VTE. We developed a Markov model with a cycle length of 1-week to simulate costs and quality-adjusted life years (QALYs) for patients receiving either apixaban or usual care over 6 months (Figure 1).
To estimate the baseline time varying risk of VTE among ambulatory cancer patients undergoing chemotherapy, we created pseudo patient-level data from survival curves reported for patients in the placebo arm of the AVERT trial using 'WebPlotDigitizer'. We fitted parametric survival models to the patient-level data points to extrapolate VTE risk beyond the trial follow-up period (median follow-up 183 days). The best model was selected based on a visual inspection and the Akaike Information Criterion. The relative risk of VTE, clinically relevant non-major bleeding, and major bleeding (using the International Society of Thrombosis and Haemostasis criteria) as a result of apixaban was obtained from the AVERT trial using the on-treatment analysis. We conducted a targeted literature search to obtain the risk of complications among cancer patients receiving low-molecular-weight heparin for the initial treatment and secondary prevention of VTE using a meta-analysis technique. Hazard ratio for increased risk of death due to cancer was estimated as a weighted average of the age-standardized mortality rate by tumor type, based on the proportion of patients with each tumor type in the AVERT trial. Costs were obtained from published Canadian sources. Baseline health utility values for patients on chemotherapy and in remission were calculated as a weighted average of utility values by tumour type, also based on the proportion of patients with each tumour type in the AVERT trial. Utility values for chemotherapy and remission for each tumour type, as well as event specific disutility values, were obtained from the published literature. Both costs and QALYs were discounted using an annual rate of 1.5%, as recommended by the Canadian Agency for Drugs and Technologies in Health. We conducted deterministic and probabilistic sensitivity analyses to assess robustness of study findings.
Results:
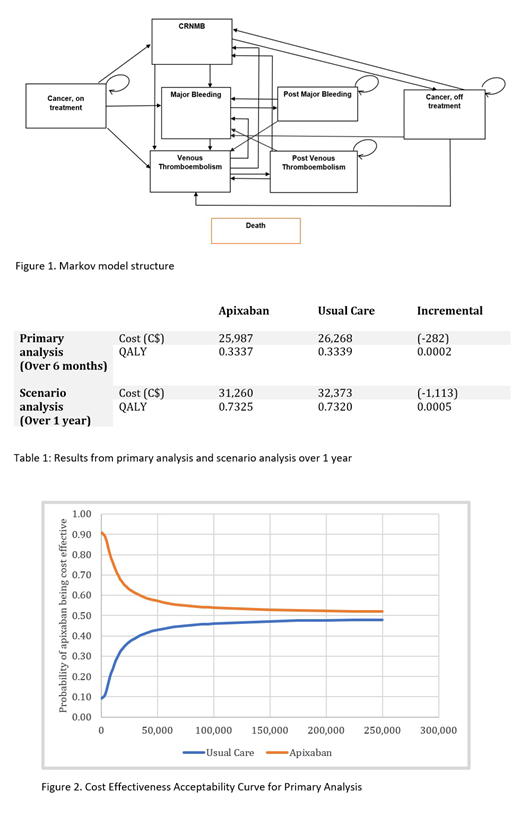
Over a 6-month period, apixaban was associated with a lower health system cost (C$25,987 vs C$26,268) and a slight increase in QALYs (0.3339 vs 0.3337) compared to usual care (Table 1). The probability that apixaban was cost-saving compared to usual care was 90%; however, this probability decreased with the greater willingness to pay (WTP) values partly due to the high uncertainty in the difference in QALYs. At a WTP threshold of C$50,000/QALY, the probability of apixaban being cost effective was 57% (Figure 2).
Over 1 year, apixaban reduced health care system costs by C$1,113 and improved QALYs by 0.0005 units compared to usual care. At a WTP threshold of C$50,000/QALY, the probability of apixaban being cost effective increased to 70%.
Our results were robust to the change in time horizon; however, they were more sensitive to the relative risk of VTE, the relative risk of major bleeding, the costs amassed in the post-VTE period, and the treatment cost of acute VTE. Probabilistic sensitivity analysis indicated a high-level uncertainty around cost effectiveness estimates, which may be driven by the wide confidence intervals around estimates for relative risk of complications in patients receiving thromboprophylaxis with apixaban.
Conclusion:
From a publicly funded health system's perspective apixaban is a cost saving option for thromboprophylaxis among ambulatory cancer patients initiating chemotherapy.
Wells:BMS/Pfizer: Honoraria, Research Funding; Bayer: Honoraria; Sanofi: Honoraria; Daiichi Sankyo: Honoraria. Carrier:Servier: Honoraria; Bayer: Honoraria; Pfizer: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Leo Pharma: Honoraria, Research Funding.
Apixaban can be used as postoperative prophylaxis of DVT/PE and for treatment of DVT/PE. We performed a cost-utility analysis of apixaban 2.5mg BID for the primary thromboprophylaxis of ambulatory cancer patients initiating chemotherapy, at intermediate-high risk of venous thromboembolism.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal