Introduction: Cytomegalovirus (CMV) is a leading cause of morbidity following allogenenic hematopoietic stem cell transplant (HSCT). Letermovir (LTV), an orally available antiviral drug which inhibits the CMV-terminase complex, was recently approved for CMV prophylaxis in CMV-seropositive HSCT recipients due to its ability to significantly reduce the risk of clinically significant CMV infection and its favorable toxicity profile. In the pivotal phase 3 study, subgroup analysis suggested increased benefit of LTV in patients at higher risk for CMV infection (i.e. unrelated or haploidentical donor (HID) vs. matched related donor), however HID transplants represented only 16% of the study population1. Therefore, we conducted a retrospective analysis of CMV reactivation rates, before and after the initiation of routine LTV prophylaxis, to determine the real-world safety and efficacy of LTV in an unselected group of CMV-seropositive high risk HSCT recipients, including a large number of HID transplants.
Methods: We conducted a retrospective review of 106 consecutive CMV-seropositive high risk allogeneic HSCT recipients between 2017 and 2019. We compared the incidence of CMV infection immediately prior to the initiation of routine LTV prophylaxis in high risk transplant recipients (pre-LTV) (n=41) to that occurring after the initiation of LTV prophylaxis (post-LTV) (n=63). HSCT recipients were considered high risk if they had received at least one of the following: transplant from a haploidentical donor, matched unrelated donor, umbilical cord blood donor source or received anti-thymocyte globulin. CMV infection was defined as the need for pre-emptive therapy or documented CMV disease. The cumulative incidence (CI) of CMV infection at 100 days and 180 days were calculated to accommodate death as a competing risk. We used the Wald test to compare the CI at 100 and 180 days between the two cohorts.
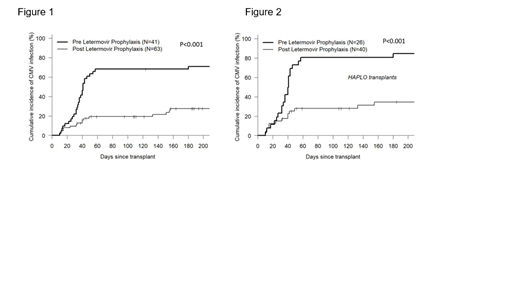
Results: Baseline characteristics of the pre- and post-LTV cohorts were similar, with HID transplants making up the majority of HSCTs in both groups, 65% and 64% respectively. We found a significantly lower CI of CMV infection at both 100 and 180 days in the post-LTV cohort when compared to the pre-LTV cohort (19.4% vs. 68.3% and 27.6% vs. 71% respectively; p<0.001) (Fig. 1). Despite lower CMV incidence following LTV prophylaxis, there was no significant difference in median time to CMV infection when compared to patients not receiving LTV prophylaxis (median [range] 40 [10, 243] vs. 36 [10, 180] days, p=0.72). The CI of CMV disease was 1.6% in the post-LTV cohort vs. 7.3% in the pre-LTV cohort (p=0.186). No significant differences were observed in any other outcome variable including overall survival, non-relapse mortality, relapse, acute graft-versus-host disease (GVHD) or time to neutrophil or platelet recovery. A preplanned subset analysis limited to HID transplant recipients (Fig. 2) again demonstrated a significant decrease in CMV infection in the post-LTV cohort at 100 and 180 days (27.9% vs. 80.8% and 34.6% vs. 84.6% respectively; p<0.001).
Conclusion: This single center analysis confirms the benefit of LTV prophylaxis in reducing the risk of clinically significant CMV infection in unselected high risk CMV-seropositive HSCT patients, including a substantial number of HID transplant recipients. We found no significant impact of LTV prophylaxis on any other transplant outcome including hematologic engraftment, GVHD, relapse or mortality. In contrast to the pivotal phase 3 study, we saw few CMV infections occurring past day 100 after discontinuation of LTV prophylaxis. Future planned analyses will include comparisons of antiviral usage and associated toxicities (i.e. cytopenias), overall treatment charges and hospitalization/resource utilization.
LaPorte:Merck: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal