Introduction- Graft-versus-host disease (GVHD) is a major complicating factor in determining morbidity and mortality following allogeneic stem cell transplant. To prevent GVHD, our institution administers calcineurin inhibitors and low doses of methotrexate (MTX) (5-15 mg/m2) on days +1, +3, +6, +11 for GVHD prophylaxis. While MTX is an efficacious agent in preventing GVHD, one of the undesirable adverse effects, which can be a dose limiting toxicity, is mucositis.
Proton pump inhibitors (PPIs) are used for the treatment of gastroesophageal reflux disease, pyrosis, and/or peptic ulcer disease. PPIs have been shown to inhibit the ATP-binding cassette drug transporter Breast Cancer Resistance Protein's (BCRP/ABCG2) ability to excrete methotrexate into the urine in a concentration-dependent manner. Delayed excretion of methotrexate can lead to adverse events such as renal impairment, increased severity of mucositis, and increased infection rates. High dose MTX, defined as 500mg/m2 or more, has been shown to have delayed excretion when administered concomitantly with PPIs. The purpose of this retrospective, single center study is to assess the clinical outcomes of patients receiving low dose MTX and PPIs compared to MTX and no PPI for GVHD prophylaxis.
Patients and Methods- A single-site institutional review board exempt retrospective analysis of patients 18 years of age or older who underwent allogeneic stem cell transplantation from 2013 to 2018 at our institution. Only patients that received methotrexate for GVHD prophylaxis were included. Cases were identified from clinical databases at our institution and were divided into two cohorts, those on PPIs, and those not on PPIs (no-PPI). Chart review was performed for the clinical variables of interest. The primary endpoint was grade of mucositis in each cohort. Secondary endpoints were total parenteral nutrition (TPN) use, need for patient controlled analgesia (PCA), leucovorin use, time to engraftment of absolute neutrophil count (ANC) and hospital length of stay (LOS). Lab values for analysis were assessed at admission and days +1/+5/+15.
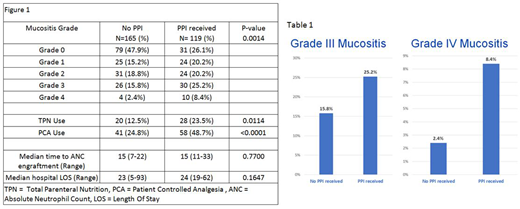
Results- 284 cases were identified, 119 (42%) patients were on a PPI at the time of MTX administration. The median age of patients on PPI was younger than those not on PPI (60 vs 54 p <0.0001). There were no differences in sex, BSA, conditioning regimen intent (ie myeloablative vs reduced intensity), dose of MTX, degree of match, or stem cell source. Elevation in serum creatinine and liver function tests were similar in both cohorts prior to and during MTX administration. Patients on PPI had a higher incidence of grade 1-4 mucositis compared to no-PPI (Table 1) and a higher percentage of grade 3-4 mucositis (Figure 1). Patients on PPIs had a higher incidence of TPN use, need for PCA, but no increase in hospital LOS. Patients on PPI had higher use of leucovorin than those not on PPI (81.2% vs 58.2% p <0.0001). Use of PPI did not increase the time of ANC engraftment.
Conclusions- The concurrent use of PPIs and MTX was associated with a higher incidence and severity of mucositis. PPIs were also shown to increase the need for PCA, and TPN. PPI use did not increase the average length of hospitalization. This study was limited by retrospective review. Grading of mucositis was based on clinician assessment and documentation. While the indications for PPI were not readily known, this study raises the question if PPIs should be discontinued or patients transitioned to a histamine blocker prior to MTX administration to improve severity of mucositis and reduce costs associated with TPN/PCA use. Additional studies are needed in order to further characterize the association of PPI with MTX and its impact on clinical outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal