
Background: Standard treatment for catheter-associated upper extremity deep vein thrombosis (UEDVT) is anticoagulation. If catheter removal is otherwise indicated, it is unknown if catheter removal close to the time of initiation of anticoagulation is associated with a higher incidence of pulmonary embolization.
Methods: A multicenter retrospective cohort study was performed at 8 participating institutions through the Venous thromboEmbolism Network US (VENUS). ICD-9/10 codes were used to identify patients with hematologic malignancies and upper extremity deep vein thrombosis (UEDVT) from 1/1/2010 through 12/31/2016. Identified patients underwent medical record review to verify diagnostic codes and determine if a catheter was associated with the upper extremity DVT and assess for outcomes. Patients were excluded if the UEDVT was not catheter provoked or if there were associated lower extremity DVT and/or pulmonary emboli. The anticoagulant start and finish date as well as the timing of the catheter removal, total follow up, and death were recorded. Patients started on anticoagulation at the time of their diagnosis were divided into two groups: 1) anticoagulation with delayed (> 48 hrs) or no catheter removal and 2) anticoagulation with early catheter removal (< 48 hrs). Outcomes were also assessed in patients with no anticoagulation initiation but catheter removal as the only treatment. The primary outcome was clinically identified pulmonary emboli within 7 days and the secondary outcome was pulmonary emboli or death from any cause within 7 days. Baseline characteristics were compared between groups using Χ2 for categorical variables, 2-tailed t-tests for continuous variables, and Wilcoxon rank-sum for nonparametric continuous variables. Fisher's exact test was used to evaluate the primary and secondary outcomes.
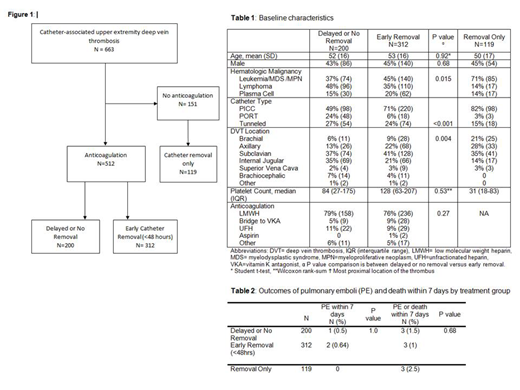
Results: 663 patients with hematologic malignancies and isolated catheter-associated UEDVT underwent chart review. 512 patients were treated with anticoagulation of which 312 underwent early catheter removal while 200 had delayed or no catheter removal (Figure 1). 151 patients received no anticoagulation and 119 underwent catheter removal alone as the treatment for the DVT. Among patients who were treated with anticoagulation, the mean age was 52.6 years and 44% were male; age and sex did not differ between patients with early vs. delayed or no catheter removal (Table 1). Type of hematologic malignancy, type of central catheter, and DVT location were significantly different between groups. Patients with PICC lines were more likely to have early catheter removal (71% vs. 49%). The median platelet count was not significantly different among patients treated with anticoagulation, but was lower in patients treated with catheter removal only. Most patients were initially treated with low molecular weight heparins (LMWH) and anticoagulation treatment did not differ between groups. Pulmonary emboli within 7 days occurred in 2 patients (0.64%) with early catheter removal compared to 1 patient (0.5%) with delayed or no removal (p=1.0). Pulmonary emboli or any cause death within 7 days occurred in 3 patients (1.0%) with early removal compared to 3 patients (1.5%) with delayed or no removal (p=0.68). In patients treated with catheter removal only (no anticoagulation), there were no pulmonary emboli within 7 days and 3 deaths. All 3 patients with pulmonary emboli within 7 days had PICC lines and leukemia/MDS and the sites of most proximal DVT involvement were brachiocephalic veins (2 patients) and subclavian vein (1 patient).
Conclusions: In patients with hematological malignancy and catheter-associated UEDVT, removal of catheters within 48 hours was not associated with increased risk of pulmonary emboli compared to delayed or no removal.
Billett:Albert Einstein College of Medicine: Patents & Royalties: Patent application pending for NETs AI software. Gaddh:Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Hema Biologics: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC: Membership on an entity's Board of Directors or advisory committees. Oo:Medical Education Speakers Network: Honoraria; Janssen and Janssen: Other: Research: site co-investigator . Jaglal:NOVARTIS: Consultancy. Streiff:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Daiichi-Sankyo: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Portola: Consultancy, Honoraria; Roche: Research Funding. Baumann Kreuziger:Vaccine Injury Compensation Program: Consultancy; CSL Behring: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal