Background: Chimeric antigen receptor T-cells (CAR-T) targeting CD19 have shown clinical efficacy in high-risk B-cell lymphomas, which has led to approval of 2 such therapies (axicabtagene ciloleucel and tisagenlecleucel) for large B-cell lymphoma after 2 lines of treatment. Despite the promising results, complete remission (CR) is achieved in ~ 50% of patients, and with longer follow-up progression-free survival is around 40%. Therefore, finding effective treatments for high-risk B-NHLs remains an unmet need. CD20 is a proven therapeutic target for B-Cell Non-Hodgkin Lymphomas (B-NHL), supported by previously approved naked and radiolabeled anti-CD20 monoclonal antibodies and a number of studies investigating novel bispecific antibodies targeting this antigen. CD20-targeted CAR-T is another potential adoptive immunotherapy option that could be utilized in combination or sequentially before or after CD19 CAR-T, depending on efficacy. Here, we present our ongoing phase I/II clinical trial investigating safety and efficacy of CD20 CAR-T for high-risk B-NHLs (NCT03277729).
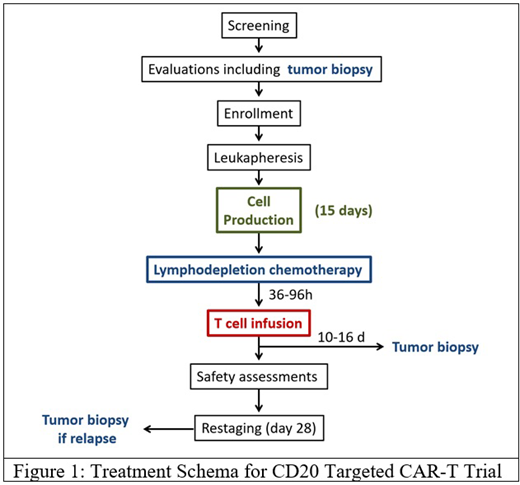
Methods: MB-106 is a fully human third-generation CD20 targeted CAR-T with both 4-1BB and CD28 costimulatory domains. In the phase I portion of the study we use a continual reassessment method dose escalation design to find the maximally tolerated dose. Lymphodepletion (LD) chemotherapy consists of fludarabine and cyclophosphamide. Patients (pts) will undergo a mandatory biopsy before LD to confirm the diagnosis and CD20 expression. A repeat research biopsy will be done between 10-16 days after CAR-T infusion (Figure 1). Except for the first patient of each dose cohort, treatment is given in the outpatient setting (Fred Hutch/Seattle Cancer Care Alliance) and pts will only be admitted to the University of Washington Medical Center if clinically indicated after CAR-T infusion. Response to treatment will be assessed on day 28 using Lugano criteria. Patients with relapsed or refractory CD20 positive B-NHL are eligible, including but not limited to DLBCL, primary mediastinal lymphoma, follicular lymphoma or other indolent histologies, and mantle cell lymphoma. Prior treatment with CD19 CAR-T is permitted as long as there is evidence of B-cell recovery in peripheral blood (≥ 20 B cells/µl) as a functional test to rule out persistent CD19 CAR-Ts. Patients need to meet standard organ function criteria and have adequate blood counts (ANC >750, Hb >8.5, Plts >50,000). Patients with significant neurologic conditions, active CNS lymphoma, or need for systemic immunosuppressive therapy are excluded from the study.
Shadman:Mustang Bio: Research Funding; Verastem: Consultancy; Genentech: Consultancy, Research Funding; Atara Biotherapeutics: Consultancy; Pharmacyclics: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Celgene: Research Funding; Sound Biologics: Consultancy; TG Therapeutic: Research Funding; Acerta Pharma: Research Funding; Sunesis: Research Funding; Gilead: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; BeiGene: Research Funding; Astra Zeneca: Consultancy. Gopal:Seattle Genetics, Pfizer, Janssen, Gilead, Sanofi, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Takeda, Compliment, Asana Bio, and Incyte: Honoraria; Seattle Genetics, Pfizer, Janssen, Gilead, Sanofi, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Takeda, Compliment, Asana Bio, and Incyte.: Consultancy; Teva, Bristol-Myers Squibb, Merck, Takeda, Seattle Genetics, Pfizer, Janssen, Takeda, and Effector: Research Funding. Smith:Pharmacyclics: Research Funding; Genentech: Research Funding; Ignyta (spouse): Research Funding; Portola Pharmaceuticals: Research Funding; Bristol-Myers Squibb (spouse): Research Funding; Ayala (spouse): Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta Pharma BV: Research Funding; Merck Sharp & Dohme Corp: Consultancy, Research Funding; Incyte Corporation: Research Funding; Seattle Genetics: Research Funding; Denovo Biopharma: Research Funding. Lynch:Juno Therapeutics: Research Funding; Rhizen Pharmaceuticals S.A: Research Funding; Takeda Pharmaceuticals: Research Funding; Johnson Graffe Keay Moniz & Wick LLP: Consultancy; Incyte Corporation: Research Funding; T.G. Therapeutics: Research Funding. Ujjani:Pharmacyclics: Honoraria; PCYC: Research Funding; Gilead: Consultancy; Astrazeneca: Consultancy; Atara: Consultancy; Genentech: Honoraria; AbbVie: Honoraria, Research Funding. Turtle:T-CURX: Membership on an entity's Board of Directors or advisory committees; Humanigen: Other: Ad hoc advisory board member; Eureka Therapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Precision Biosciences: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Caribou Biosciences: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Allogene: Other: Ad hoc advisory board member; Juno Therapeutics: Patents & Royalties: Co-inventor with staff from Juno Therapeutics; pending, Research Funding; Nektar Therapeutics: Other: Ad hoc advisory board member, Research Funding; Kite/Gilead: Other: Ad hoc advisory board member; Novartis: Other: Ad hoc advisory board member. Yeung:Pfizer: Research Funding; OBI Pharmaceutical: Research Funding; Merck: Consultancy; DiaCarta: Consultancy. Sersch:Mustang Bio: Employment. Maloney:Juno Therapeutics: Honoraria, Patents & Royalties: patients pending , Research Funding; Celgene,Kite Pharma: Honoraria, Research Funding; BioLine RX, Gilead,Genentech,Novartis: Honoraria; A2 Biotherapeutics: Honoraria, Other: Stock options . Till:Mustang Bio: Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal