Incidence and risk factors associated with Infection after Chimeric Antigen Receptor T Cell therapy for relapsed/refractory B-cell malignancies
Feng Zhu, Yongxian Hu, Guoqing Wei, Wenjun Wu, He Huang
Background:Patients with relapsed/refractory (R/R) B-cell malignancies have a very poor prognosis although novel drugs have been developed recently. Chimeric antigen receptor T cells (CAR-Ts) have been successful in improving treatment outcomes for B-cell malignancies. As CAR-T cell therapy becomes more widely applied in clinical practice, as well as data from long-term follow-ups are piled up, CAR-T unique toxicities have been increasingly identified including cytokine release syndrome (CRS), B-cell aplasia, CAR-T-cell-related encephalopathy syndrome (CRES) etc. To date, infection as another common and severe complication which was frequently occurred in patients treated by chemotherapy, is rare recognized after CAR-T cell treatment.
Study Design and Methods:
We evaluated infection events occurring between days 0-28 and 29-180 in 92 patients with 113 CAR-T cell infusion in a phase 1/2 study from July 2015 to May 2019. The cohort included patients with acute lymphoblastic leukemia (ALL, n=58) and non-Hodgkin lymphoma (NHL, n=34). Patients were censored on the date of new antitumor therapy, last clinical contact at our hospital, or death. The survival time was dated from the day of the first CAR-T-cell infusion (day 0) to death or July 1st 2019. Infections were recorded if there was a microbiologic diagnosis, The patients with typical clinical symptoms including radiographic evidence without CAR-T cell expansion were identified as infected ,though their microbiologic results were negative.
Results
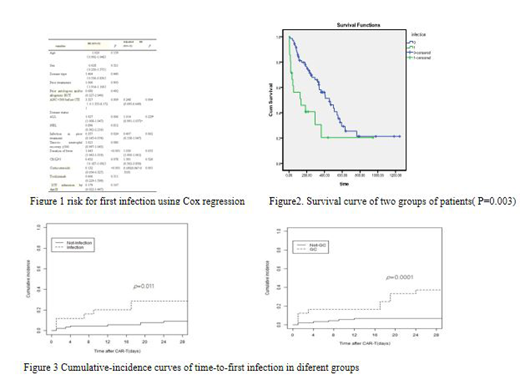
There were 15 infections within 28 days after CAR-T cell infusion with an infection density of 0.5 infections for every 100 days-at-risk. There was a lower infection density of 0.1 between days 29-180. The first infection was identified at a median time of 7 days (range, 1-24 days) after CAR-T-cell infusion; 60% of the first infections occurred within the first 10 days. Bacterial infections predominated including 4 patients with bloodstream infections. Viral infections were the second most frequent infection with 4 events in early periods, while viral infections were more often than bacteria in late periods. The incidence of infections in our study was much lower than other CAR-T clinical trials as recently reported. We used multivariable Cox regression to evaluate risk factors for infection. ANC < 500 cells per mm3 before CAR-T cell infusion and corticosteroid administration for cytokine release syndrome treatment were 2 independent risk factors associated with infection after CAR-T therapy in a multivariable analysis (Figure 1). Finally we divided the patients into two groups based on infection or not, and found that patients with infection had shorter survival time than those without infection with statistical difference (P=0.003) (Figure 2).Cumulative-incidence curves of the time to infection within 28 days after CAR-T-cell infusion stratified by different groups are depicted in Figure 3. These findings indicate that patients who had been infected before and treated by corticosteroid administration were at higher risk of infection after CAR-T-cell immunotherapy.
Conclusion:
ANC < 500 cells per mm3 before CAR-T cell infusion and corticosteroid administration for cytokine release syndrome treatment were 2 independent factors associated with infection. While infectious complications have an important role in prognosis of patients with R/R ALL and NHL after CAR T-cell therapy. Based on our observations of the frequency and timing of infections, we should pay attention to bacteria infection in the early period (day0-day28) and viral infection in the late period (day29-day180). For the high risk patients, given effective anti-infective treatment may improve their prognosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal