R.S.K and B.N.L contributed equally to this study.
The induction of tolerance towards pancreas autoantigens is a promising approach in the treatment of type 1 diabetes mellitus (T1DM). This goal can be partially attained by immuno-ablation followed by autologous HSCT which mitigates the immune reaction leading to diabetes, at least temporarily, if performed soon after diagnosis. However, most T1DM patients transplanted with autologous HSCT eventually relapse (E Snarski et al., Bone marrow Transplantation; 2016; 51, 398-402). Thus, developing methodologies for allogeneic HSCT, to provide a durable non-autoimmune TCR repertoire, could be a promising treatment approach provided that such protocols can safely achieve donor type chimerism. Accordingly, transplantation of T cell-depleted allogeneic HSCT (TD-HSCT) under mild conditioning, associated with minimal toxicity and reduced risk of GVHD, offers an attractive therapeutic option. However, overcoming rejection after reduced conditioning in T1DM represents a major challenge.
We previously demonstrated in wild type mice that rejection of TD-HSCT can be prevented using donor-derived veto cells. Here, we show proof of concept of the safety and efficacy of veto cell mediated non-myeloablative mismatched allogeneic TD-HSCT, in the established NOD mouse T1DM model. Veto activity, first defined by Miller (Miller, R. G; Nature; 1980; 287; 544-54), is based on the ability of specific cell populations to attack host CTL-precursors (CTLp) that are directed against the antigens presented by the veto cells themselves. This response spares cells that are not targeted against the veto cells, including those recognizing pathogens. Among different veto cell populations described in the literature, central memory CD8 T cells exhibit the most robust veto activity upon transplantation, but are also endowed with marked GvH activity. We overcame GvH reactivity by expanding naïve or memory CD8 T cells against 3rd party MHC or viral antigens, under culture conditions favoring expression of the central memory phenotype. Such anti-3rd party central memory CD8 T cells (Tcm) are endowed with marked veto activity, while effectively depleted of GVH reactivity in fully mis-matched recipients (Reviewed in Reisner Y, Or-Geva N. Semin. Hematol. 2019; 56(3): 173-182).
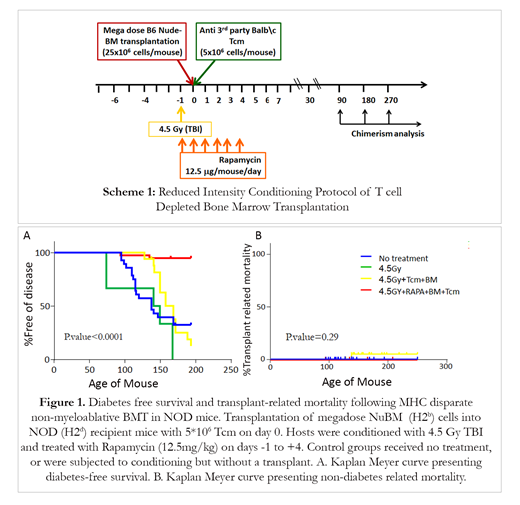
In this study, Tcm veto cells were generated from splenocytes obtained from Balb/c donors (H2d) cultured against irradiated third-party splenocytes (FVB; H2q), under cytokine deprivation. The selective expansion of CD8 mouse T cells against 3rd party stimulators under these conditions leads to selective 'death by neglect' of bystander anti-host T cell clones that could mediate GvHD; such cells are further diluted out by subsequent expansion of anti-3rd party T clones during further culture in the presence of IL15. In addition to causing selective loss of GvH reactive T cells, these culture conditions induce a central memory phenotype shown to be crucial for robust veto activity in vivo. To evaluate the safety and efficacy of such a transplant for diabetes therapy, 8 week old NOD mice (before diabetes onset) were treated with 4.5 Gy TBI conditioning at day -1, anti-3rd party veto Tcm and megadose nude bone marrow (NuBM) on day 0, and Rapamycin treatment from day -1 to day +4 (Scheme 1). Controls were untreated or received conditioning with no transplant. In four independent experiments (n= 35 NOD mice in the transplanted group), high chimerism from 83.5% to 99.6 % was found in all transplanted mice at 6 months post-transplant. Notably, with a follow-up of 200 days, 72.4% mice in the untreated group, and 96% in the conditioned group died of diabetes, while only 8.5% diabetes-associated mortality (Fig.1A) and no GvHD or other transplant-related mortality was observed in the transplanted mice (Fig.1B). Our results demonstrate a proof of concept for the safety and efficacy of non-myeloablative allogeneic TD-HSCT in type 1 diabetes. A clinical protocol testing the safety and efficacy of anti-3rd party veto cells in the context of low toxicity non-myeloablative TD-HSCT in hematological malignancies is commencing at MD Anderson Cancer Center. If successful, our results support further extension of this platform to autoimmune diabetes and other autoimmune diseases.
Lustig:Yeda Ltd.: Patents & Royalties. Reisner:Cell Source, Inc.: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal