
Introduction:
Reduced-intensity and non-myeloablative (RIC/NMA) conditioning regimens are frequently used in alloHCT for NHL, because they are associated with decreased non-relapse mortality (NRM) risk in comparison with myeloablative conditioning regimens and allow older patients and patients with comorbidities to receive alloHCT. However, the optimal RIC/NMA regimen in allo-HCT for NHL is not known. Using the CIBMTR database we compared the transplant outcomes between the commonly used RIC/NMA regimens in NHL.
Methods:
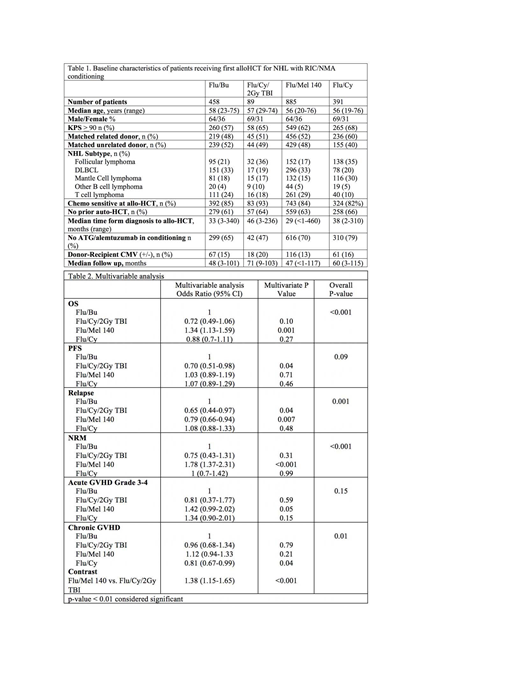
1823 adult (≥18 years) NHL patients in CIBMTR registry undergoing alloHCT using matched related or unrelated donors, between 2008-2016 were included. Analysis was limited to patients receiving four commonly used RIC/NMA regimens: fludarabine/ i.v. busulfan (6.4mg/kg) (Flu/Bu), fludarabine/melphalan (140mg/m^2) (Flu/Mel 140), fludarabine/cyclophosphamide (Fly/Cy) and Flu/Cy with 2Gy Total Body Irradiation (Flu/Cy/TBI). Graft-versus-host disease (GVHD) prophylaxis was limited to calcineurin inhibitor-based regimens. Patients who received post transplantation cyclophosphamide for GVHD prophylaxis were excluded. The primary outcome was overall survival (OS). Secondary outcomes included cumulative incidence of NRM, relapse, progression-free survival (PFS) and cumulative incidence of acute and chronic GVHD. Probabilities of OS and PFS were calculated using the Kaplan-Meier estimator. Multivariable regression analysis was performed for GVHD, relapse/progression, NRM, PFS, and OS. Covariates with a p<0.01 were considered significant.
Results:
The study cohort was divided into 4 groups; Flu/Bu (n=458), Flu/Cy/TBI (n=89), Flu/Mel 140 (n=885) and Flu/Cy (n=391). The baseline characteristics are shown in Table 1. The 4 cohorts were comparable with respect to median patient age, gender, donor type, remission status at alloHCT, and use of prior auto HCT. There was no interaction between NHL histological subtype and type of conditioning regimen. Results of multivariate analysis are shown in Table 2. The Flu/Mel 140 regimen was associated with a higher NRM (HR 1.78, 95% CI 1.37-2.31; p<0.001) when compared to Flu/Bu. Although the risk of relapse with Flu/Mel 140 was lower when compared to Flu/Bu (HR 0.79, 95% CI 0.66-0.94); p=0.007), this did not result in an improvement in PFS. Moreover, the Flu/Mel 140 cohort had an inferior OS (HR 1.34, 95% CI 1.13-1.59; p<0.001) when compared to Flu/Bu. There was no significant difference in terms of OS, PFS, relapse and NRM between Flu/Bu, Flu/Cy and Flu/Cy/TBI. There was no difference in risk of grade 3-4 acute GVHD across the four cohorts and compared to Flu/Cy/TBI, Flu/Mel 140 had a higher risk of chronic GVHD (HR 1.38, 95% CI 1.15-1.65; p<0.001). Four year adjusted PFS was 38% for Flu/Bu, 51% for Flu/Cy/TBI, 39% for Flu/Mel 140 and 35% for Flu/Cy (p=0.07). Four year adjusted OS was 58% for Flu/Bu, 67% for Flu/Cy/TBI, 49% for Flu/Mel 140 and 63% for Flu/Cy (p<0.001). Disease relapse was the most common cause of death across all 4 cohorts.
Conclusion:
This is the largest analysis comparing the impact of various RIC/NMA conditioning regimens on the outcomes of NHL patients undergoing alloHCT. We report that the choice of RIC/NMA conditioning regimen significantly impacted OS. The most commonly used conditioning regimen, Flu/Mel 140 was associated with a higher NRM and an inferior OS. The Flu/Bu, Flu/Cy, and Flu/CY/TBI conditioning regimens appear to provide comparable OS.
Ghosh:Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; SGN: Consultancy, Honoraria, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Research Funding; Genentech: Research Funding; Forty Seven Inc: Research Funding; AstraZeneca: Honoraria, Speakers Bureau. Kharfan-Dabaja:Pharmacyclics: Consultancy; Daiichi Sankyo: Consultancy. Sureda:Amgen: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria; Sanofi: Honoraria; Takeda: Consultancy, Honoraria, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Novartis: Honoraria; Roche: Honoraria. Hamadani:Merck: Research Funding; ADC Therapeutics: Consultancy, Research Funding; Celgene: Consultancy; Otsuka: Research Funding; Medimmune: Consultancy, Research Funding; Takeda: Research Funding; Pharmacyclics: Consultancy; Janssen: Consultancy; Sanofi Genzyme: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal