BACKGROUND: In multiple myeloma (MM), deep responses have been associated with improvements in progression-free survival (PFS) and overall survival (OS). Response kinetic data, including renal response times, which are highly important for patients with renal impairment, are infrequently reported. We analyzed the association between depth of response, including minimum residual disease (MRD) negativity, plus response kinetics and long-term outcomes, using data from the randomized, open-label, phase 3 ICARIA-MM study. ICARIA-MM evaluated the anti-CD38 monoclonal antibody isatuximab (Isa) in combination with pomalidomide and dexamethasone (Pd) versus Pd in patients with relapsed/refractory MM (RRMM) after ≥2 prior lines of therapy (NCT02990338).
METHODS: All patients received standard doses of Pd and those randomized to the Isa-Pd group received 10 mg/kg IV on days 1, 8, 15, and 22 (cycle 1), and days 1 and 15 in subsequent 28-day cycles. Depth and kinetics of response were analyzed for each treatment group. MRD was assessed at 10-5 (tested by next-generation sequencing in patients with complete response [CR] / stringent CR [sCR]). Time to tumor response, time to renal response (CRenal; improvement in estimated glomerular filtration rate (eGFR), using the MDRD formula, from <50 mL/min/1.73m² at baseline to ≥60 mL/min/1.73m² at ≥1 post-baseline assessment), and time to sustained CRenal (a CRenal lasting ≥60 days) were recorded. No neutralization assay was used for patients with IgG kappa clonality.
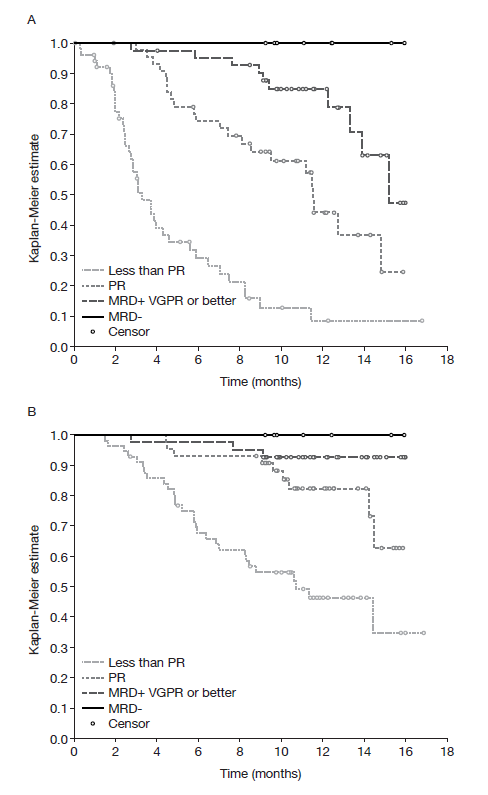
RESULTS: Overall, 307 patients were randomized to Isa-Pd (n=154) or Pd (n=153), of whom 33/142 (23.2%) and 24/145 (16.6%) patients had eGFR <50 mL/min/1.73m² at baseline. Patients had received a median of 3 prior lines of therapy (range 2-11) and 73.4% and 71.9% of patients in the Isa-Pd and Pd groups, respectively, were double refractory. Median PFS was 11.53 months with Isa-Pd and 6.47 months with Pd (hazard ratio [HR] 0.596; 95% confidence interval [CI] 0.436-0.814). Tumor responses on Isa-Pd were more frequent and deeper than on Pd. Overall response rates (ORR) were 60.4% vs 35.3% (odds ratio [OR] 2.80; 95% CI 1.72-4.56; p<0.0001); at least very good partial response rates (≥VGPR) were 31.8% vs 8.5% (OR 5.03; 95% CI 2.51-10.59; p<0.0001). As no isatuximab interference assay was performed, near-complete response rates (immunofixation remained positive) were reported: 15.6% vs 3.3% (OR 5.47; 95% CI 1.96-18.78; p=0.0002). MRD negativity rates in the ITT population were 5.2% vs 0%. Depth of response correlated with improved long-term outcomes in both arms (Figure). After a median follow-up of 11.6 months in the Isa-Pd arm, 100% of MRD-negative (MRD−) patients were progression free and alive. In the Isa-Pd arm, median PFS was longer with increased depth of response: MRD− patients, not reached (NR); ≥VGPR and MRD+, 15.21 months; partial response (PR), 11.53 months; less than PR, 3.29 months. This pattern was also observed for 1-year OS probabilities (100% > 92.9% > 82.4% > 46.4%, respectively).
Tumor responses occurred faster with Isa-Pd than with Pd. Among patients who achieved a response of ≥PR (93 in the Isa-Pd arm and 54 in the Pd arm), the median time to first response was shorter for Isa-Pd (1.1 months) than for Pd (1.9 months). Among patients who achieved a response of ≥VGPR (49 and 13 in the Isa-Pd and Pd arms, respectively), the time to first VGPR or better response was similar at 2.9 months for Isa-Pd and 3.0 months for Pd. Renal responses occurred faster in patients on Isa-Pd than on Pd. A CRenal was observed in 23/32 (71.9%) patients in the Isa-Pd arm (median time to response 3.4 weeks) and in 8/21 (38.1%) of patients in the Pd arm (median time to response 7.3 weeks). A sustained CRenal was observed in 10/32 (31.3%) patients in the Isa-Pd arm (median time to first response 2.4 weeks) and in 4/21 (19.0%) patients in the Pd arm (median time to first response 4.8 weeks).
CONCLUSION: In the heavily pretreated ICARIA population, Isa-Pd induced more frequent and faster tumor and renal responses than Pd. Depth of response, including MRD negativity, was improved with Isa-Pd and was clearly associated with better long-term survival outcomes.
Figure. PFS (A) and OS (B) by best overall response and minimal residual disease for Isa-Pd
Hulin:celgene: Consultancy, Honoraria; Janssen, AbbVie, Celgene, Amgen: Honoraria. Richardson:Sanofi: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Research Funding. Spicka:Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria; Janssen-Cilag: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Sanofi: Consultancy; Amgen: Consultancy, Honoraria; Sanofi: Consultancy; Novartis: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Le-Guennec:Sanofi: Employment. Campana:Sanofi: Employment. van de Velde:Sanofi: Employment. Beksac:Celgene: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Isatuximab is an investigational agent and has not been approved by the US Food and Drug Administration or any other regulatory agency worldwide for the uses under investigation.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal