Background
US MM-6 is a US community-based study that investigates the transition from a parenteral (btz) to an oral (ixazomib) proteasome inhibitor (PI) in NDMM to increase PI-based treatment duration and adherence, maintain QoL, and improve outcomes. Here, we report on the novel, RW aspects of the study including the use of digital devices/wearables to evaluate QoL, medication adherence, and actigraphy (average steps and sleep time/ day) in a community oncology setting, for the first 55 enrolled pts.
Methods
NDMM pts who are transplant-ineligible or transplant-delayed >24 mos and have ≥stable disease after 3 cycles of btz-based induction are being enrolled at 23 community sites. Pts receive IRd (ixazomib 4 mg, d 1, 8, 15; lenalidomide 25 mg, d 1-21; dexamethasone 40 mg [20 mg for pts aged >75 yrs], d 1, 8, 15, 22; each 28-d cycle) for up to 26 x 28-d cycles or until progression/toxicity. The primary endpoint is progression-free survival. Novel secondary and exploratory endpoints are included to capture pts' experience in the RW community setting. Electronic pt-reported outcomes (ePROs) are used to assess medication adherence and QoL, as measured by the European Organization for Research and Treatment of Cancer (EORTC) Core QoL Questionnaire (QLQ-C30), EORTC QoL Questionnaire-Multiple Myeloma Module (QLQ-MY20) for peripheral neuropathy (PN), and the Treatment Satisfaction Questionnaire for Medication (TSQM-9). Pts use wearable digital devices/smartphones to complete a monthly medication adherence survey and record actigraphy.
Results
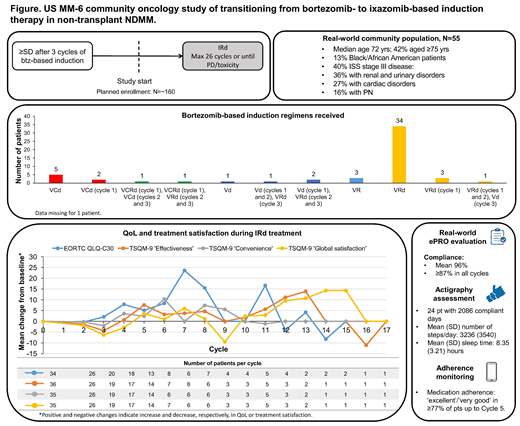
At the data cutoff of April 1 2019, 55 pts had been enrolled at 16 sites in the US; 40 were still undergoing treatment. Females comprised 53% of pts, 13% were of Hispanic/Latino ethnicity, and 13% were black/African American. Pt/disease characteristics revealed a comorbid, difficult-to-treat RW population: median age was 72 yrs (35% 65-75 yrs; 42% ≥75 yrs); 40% had International Staging System stage III disease, and 42% had lytic bone disease. Most common comorbidities at study start were hypertension (51%), anemia (44%), fatigue (42%), renal and urinary disorders (36%), gastroesophageal reflux disease (31%), and cardiac disorders (27%).
Prior to IRd treatment, the 3-cycle btz-based induction required adjusting in 11 (20%) pts: 38 (69%) pts started induction with btz-lenalidomide-dexamethasone (VRd) (1 had only 1 cycle documented, 1 de-escalated to Vd), 7 (13%) started on btz-cyclophosphomide-dexamethasone (VCd) (2 had only 1 cycle documented), 4 (7%) started on Vd (3 escalated to VRd), and 2 (4%) started on VCRd but de-escalated to VCd/VRd after 1 cycle; 3 (5%) pts received VR only (Figure).
Average compliance with completing issued ePRO questionnaires during IRd treatment was 96%, and ≥87% in all cycles (61 pts; data cutoff July 8, 2019), revealing the feasibility of ePRO collection in community pts, most of whom were elderly. During IRd treatment, there was a trend toward improved treatment satisfaction and QoL, with no increase in PN symptoms (Figure). Mean change (95% CI) from baseline on EORTC QLQ-C30 score was 5.12 (-13.79-24.02) by cycle 5 (n=13), 15.47 (-16.45-47.39) by cycle 8 (n=7), and -4.18 (-21.29-12.94) by cycle 12 (n=5). Mean change (95% CI) from baseline on TSQM-9, subscale 'effectiveness', was 7.54 (-1.84-16.91) by cycle 5 (n=14), and 11.13 (-12.82-35.08) by cycle 12 (n=3), with similar patterns for subscales 'convenience' and 'global satisfaction' (Figure). Mean change from baseline on EORTC QLQ-MY20, PN component, was between 0.0-2.0 throughout all cycles. Patients recorded their monthly medication adherence for the previous 4 weeks; 81% of evaluable pts (n=32) in cycle 1, 81% in cycle 2 (n=27), 77% in cycle 3 (n=22), 96% in cycle 4 (n=24), and 94% in cycle 5 (n=18) (n<11 [20% of pts] beyond cycle 5) reported 'excellent'/'very good' adherence.
Analysis of actigraphy data for 24 pts (2086 compliant days [≥12h of data]) (Figure) revealed normal levels of activity (Tudor-Locke 2011) and sleep durations (Coleman 2011). Updated actigraphy data will be presented.
Conclusions
Preliminary ePRO and actigraphy data from this RW community study suggest that long-term treatment with all-oral IRd has no impact on health-related QoL or on pts' lifestyle. High ePRO compliance indicates that RW studies using wearable electronic data collection devices are feasible in this mostly elderly, comorbid population, and may have a positive impact on medication adherence.
Noga:Takeda: Employment. Rifkin:Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Birhiray:Alexion: Consultancy; Bayer: Honoraria; Helsin: Honoraria; Sanofi Oncology: Speakers Bureau; Puma: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Incyte: Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Speakers Bureau; Pfizer: Speakers Bureau; Amgen: Honoraria, Speakers Bureau; BMS: Speakers Bureau; Tessaro: Speakers Bureau; Exelexis: Speakers Bureau; Kite Pharma: Honoraria; Clovis Oncology: Speakers Bureau; Lilly: Speakers Bureau; AstraZeneca: Speakers Bureau; Celgene: Honoraria; Takeda: Research Funding, Speakers Bureau; Genomic Health: Speakers Bureau; Jansen Bioncology: Consultancy, Speakers Bureau; Seattle Genetics: Honoraria; Abbvie: Consultancy, Honoraria; Coheris: Honoraria. Lyons:Texas Oncology: Equity Ownership; Amgen: Consultancy; McKesson: Other: Leadership. Whidden:Takeda: Employment. Schlossman:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Wang:Millennium Pharmaceuticals, Inc., Cambridge, MA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment. Boccia:Celgene: Speakers Bureau; Genentech: Speakers Bureau; Amgen: Speakers Bureau; AstraZeneca: Speakers Bureau; AMAG: Consultancy; DSI: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal