Background
AL amyloidosis is a plasma cell dyscrasia and the most common form of amyloidosis. It is associated with deposition of light chain amyloid, which leads to organ dysfunction, most commonly of the heart and kidneys. Treatment focuses on the reduction of the production of the monoclonal light chains and includes the off-label use of antimyeloma drugs. Their use, however, is often limited by the advanced organ dysfunction frequently encountered in these patients. Daratumumab, a monoclonal CD38 antibody has shown efficacy in both newly diagnosed and relapsed AL amyloidosis in several reports. It is usually well tolerated in patients with advanced AL amyloidosis-related heart failure and requires no dose adjustment for impaired kidney function.
Aims and Methods
The aim of this study was to retrospectively assess the efficacy of daratumumab in a single-center cohort of newly diagnosed AL amyloidosis patients. A chart review was performed on all patients treated with frontline daratumumab as an individual healing attempt at the Department of Oncology at the Medical University of Vienna between April 2017 and May 2019.
Results
A total of 14 patients (nine male, five female) were evaluable. According to the 2004 Mayo staging system one patient was stage I, four patients were stage II and nine patients were stage III, two of which were in the very high risk group IIIb (NT-proBNP > 8500 ng/L). Twelve patients produced monoclonal lambda light chains. Median number of organs involved was 2 (1-4), with heart (78.6%) and kidneys (64.3%) being the most common.
All patients received daratumumab at the dose of 16 mg/m2 for at least 3 cycles (range 3-19). Dexamethasone was reduced to 20 mg per daratumumab infusion to increase tolerability. After a median follow-up of 12.5 months (range 2.17-25.17), eleven patients were still receiving daratumumab at time of analysis. One patient with stage IIIa proceeded to heart transplant after four cycles and later underwent autologous stem cell transplantation, while two patients were lost to follow-up (one patient had to withdraw from treatment due to psychiatric comorbidities; one patient moved to a different center).
Seven patients were treated with daratumumab alone, seven patients received either additional proteasome inhibitors, additional IMIDs, or both during the course of their treatment. One patient was also briefly treated with oral cyclophosphamide. Two patients experienced infusion reaction at first administration. There was no severe toxicity leading to discontinuation observed, the most significant toxicity was susceptibility to infections for which six patients received intravenous immunoglobulins.
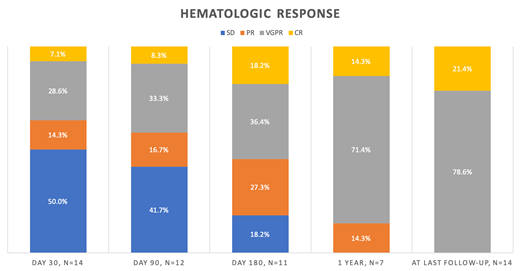
Hematologic and organ response were assessed using previously published criteria (Comenzo et al 2012). All patients showed hematologic response at last follow-up. First hematologic response was classified as PR in 50% and VGPR in 50% after a median time of 44 days (7-252). Best hematologic response was VGPR in 78.6% and CR in 21.4%. The rates remained the same to last follow-up. Median time to best response was 277 days (14-412). In the group of high and very high risk patients 77.8% achieved VGPR and two stage IIIa patients (22.2%) achieved CR at last follow-up. Cardiac response was seen in five out of eleven evaluable patients, all classified as stage IIIa, while kidney response was seen in three out of nine (33.3%) patients with kidney involvement. Median time to heart and kidney response was 281 (88-348) and 196 (62-215) days respectively.
Conclusion
Daratumumab showed high efficacy as a firstline treatment in newly diagnosed AL amyloidosis yielding a 100% objective hematologic response rate and a significant organ response rate even in high risk patients that usually have a very poor prognosis and limited treatment options. Despite the limitations inherent to any retrospective study, our data underline the role of daratumumab as a safe, effective, and tolerable treatment option in AL amyloidosis both as a monotherapy as well as a combination partner for other agents, in particular in patients with advanced stage AL amyloidosis.
Off-label use of daratumumab in AL Amyloidosis as an individual healing attempt
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal