Introduction: Survival outcome of patients with multiple myeloma (MM) is heterogeneous because of different disease- and patient-related prognostic factors as well as in treatment-related factors. A survival benefit of continuous and maintenance (C/M) therapies after induction therapy in both transplant-eligible and -ineligible patients has been demonstrated in clinical trials. However, its usefulness is still to be clarified in the patients treated in routine clinical practice. Here, we report survival outcome of patients who received C/M therapy after induction therapy by analyzing the data of a multicenter retrospective study of the Japanese Society of Myeloma.
Methods: Compiled clinical data of the patients diagnosed with MM and treated between 2013 and 2016 were analyzed retrospectively. Treatment decision whether or not to implement autologous stem cell transplantation (ASCT) was made at the discretion of a physician-in-charge. Treatment response was assessed by the investigators according to the International Myeloma Working Group uniform response criteria. Treatment decision to implement maintenance therapy after ASCT or to continue frontline treatment in non-transplanted patients was made based on the response to frontline treatment by the physician-in-charge. Patients were bifurcated using the propensity score methods in terms of age, gender, R-ISS stage, and commencement of ASCT to those who received C/M therapy and others without.
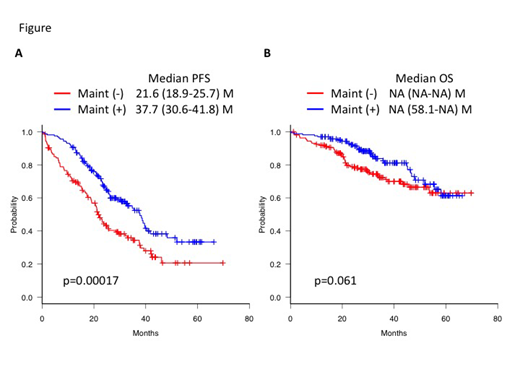
Results: A total of 771 patients were enrolled. Among them, 216 patients received frontline C/M therapy, while the remaining 555 patients did not. In 720 patients who have achieved at least stable disease after induction therapy with or without ASCT, 161 patients of C/M group and another 161 patients of non-C/M group were matched according to the baseline covariates. Patient demographics and disease characteristics were well balanced between the matched set of patients (C/M vs non-C/M) in terms of median age (years, 65 vs 65), gender (male, 52% vs 53%), and R-ISS (stage I, 19% vs 20%; stage II, 70% vs 69%; and stage III, 11% vs 11%), respectively. ASCT were performed in 54% vs 54% in either group. C/M regimens included lenalidomide (n=73), bortezomib (n=35), ixazomib (n=9), elotuzumab (n=6), thalidomide (n=5), carfilzomib (n=2), combination of proteasome inhibitors and IMiDs (n=28), and others (n=3). Median PFS was significantly prolonged in the C/M group compared with the non-C/M group (37.7 vs 21.6 months, p=0.00017, Figure A). Median PFS in each of the two groups of the transplanted and non-transplanted patients were 40.5 vs 29.5 months (p=0.0063) and 24.6 vs 11.2 months (p=0.0013), respectively. For patients who have attained a CR after the frontline therapy, there was no significant difference in median PFS between the C/M and non-C/M groups (not reached vs 37.5 months, p=0.39). However, there was a significant prolongation of median PFS in the C/M group among those who did not achieve a CR (33.0 vs 19.2 months, p=0.006). Median OS was not reached in either groups, but it appeared to be longer in the C/M group than in the non-C/M group (p=0.061, Figure B). Although median OS of both groups were similar in the transplanted patients (p=0.56), there was a significant prolongation in the non-transplanted patients (58.1 months vs not reached, p=0.024). For those who have attained a CR, there was no significant difference in median OS between the C/M and non-C/M groups (p=0.29), but for those who did not achieve a CR, there was a significant prolongation of median OS in the C/M group (58.1 months vs not reached, p=0.048). A significant beneficial impact of C/M therapy on PFS and OS was observed in the R-ISS stage II and III patients but not in the stage I patients. In multivariate analysis, R-ISS stage, CR response after frontline therapy, ASCT, and C/M therapy were significant prognostic factors for PFS, whereas R-ISS and C/M therapy were associated with prolonged OS.
Conclusions: C/M therapy had a beneficial impact on PFS and OS especially in patients with non-transplanted, non-CR response, and R-ISS stage II and III cohorts, but exerted less impact on standard-risk patients in our current clinical practice. Thus, C/M therapy is a key strategy for high-risk patients, but clarification of patients other than risk status who benefit from C/M therapy might be needed to further improve the prognosis of high-risk patients with MM.
Handa:Ono: Research Funding. Sunami:MSD: Research Funding; GSK: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; Alexion-pharma: Research Funding; Janssen: Research Funding; Takeda: Honoraria, Research Funding; Sanofi: Research Funding; Daiichi Sankyo: Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Ono: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Suzuki:Ono: Research Funding; BMS: Honoraria, Research Funding; Takeda: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Iida:Takeda: Honoraria, Research Funding; Sanofi: Research Funding; Gilead: Research Funding; Celgene: Honoraria, Research Funding; Daichi Sankyo: Honoraria, Research Funding; Kyowa Kirin: Research Funding; Abbvie: Research Funding; Novartis: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; MSD: Research Funding; Chugai: Research Funding; Janssen: Honoraria, Research Funding; Astellas: Research Funding; Teijin Pharma: Research Funding. Nishimura:Chugai-Roche Pharmaceuticals Co.,Ltd.: Consultancy; Celgene K.K.: Honoraria. Shimizu:Amgen: Consultancy; Fujimoto: Consultancy; Daiichi: Consultancy; Medical Biological Laboratory: Consultancy; Takeda: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal