Background: Systemic immunoglobulin light chain-associated amyloidosis (AL) is a rare disorder associated with plasma cell dyscrasias and the production of monoclonal free light chain proteins. Pathology results from the extracellular deposition of proteinaceous (amyloid) fibrils in association with proteoglycans and other serum derived proteins. The amyloid accumulates in abdominothoracic organs, notably the heart, liver, spleen, kidneys, as well as peripheral nerves leading to organ dysfunction and significant morbidity. At present, there are no radiotracers approved in the US for the non-invasive quantitative measurement of systemic AL amyloid disease.
We have developed a synthetic polybasic peptide radiotracer, designated 124I-p5+14, which has been shown, in preclinical assays, to bind many forms of amyloid, including AL, ATTR, and ALECT2 (Wall, J.S. et al. (2015) Molecules, 20, 7657). The peptide binds, through multivalent electrostatic interactions, to the hypersulfated heparan sulfate glycosaminoglycans and potentially to the fibrillar constituents of amyloid deposits. Using SPECT and PET imaging of murine systemic amyloidosis, we have demonstrated the specific interaction of radioiodinated peptide p5+14 with amyloid in abdominothoracic organs and tissues. No detectable binding of p5+14 with amyloid-free tissues was observed. In light of these data, p5+14 was prepared for a first-in-human PET imaging study using iodine-124-labeled peptide (NCT 03678259).
Methods: Patients >18 years of age with biopsy proven amyloidosis with adequate renal function and not requiring heparin therapy are eligible. Subjects receive <2 mg of 124I-p5+14 (<2 mCi) administered as a single IV bolus. PET/CT images for the initial cohort (n = 3) were acquired at 25 min, 50 min, 2 h, 3 h, 6h, 24 h and 48 h post injection. The second cohort of patients are imaged at ~6 h and 24 h post injection. Image data are acquired using a Biograph 16 PET/CT scanner using a low dose CT and 5 min bed positions for PET imaging to cover the full CT field of view. The primary endpoint includes safety and dosimetry estimation with a secondary endpoint of efficacy (biodistribution and organ-specific retention) of 124I-p5+14. Patients receive follow up contact by study staff on days 9 and 28 for safety and symptom assessment.
Results: To date, 10 patients have been dosed and evaluated. No serious adverse events of any grade were noted. Organ-specific and whole body effective dosimetry were calculated using time activity curves generated from the initial cohort. The gender-averaged mean whole body effective dose attributed to 124I-p5+14 injection was estimated to be ~0.5 mSv/MBq. Blood pool clearance of the 124I-p5+14 was analyzed using a two phase exponential equation which yielded an excellent fit to the data (R2 > 0.99 for all data), with estimated fast half-life values of 11.8 min - 22.4 min, and a corresponding elimination half-life of between 667.8 min and 746.7 min.
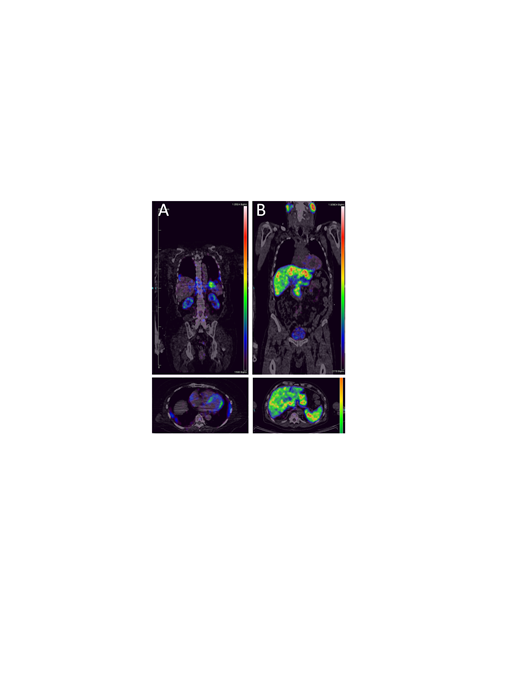
Analysis of PET images indicated retention of 124I-p5+14 in the heart, kidneys, liver, spleen, pancreas, bone marrow, lung, and adrenal gland of AL patients (Fig. 1 (A) Cardio-renal AL, and (B) Hepatosplenic AL amyloidosis). Cardiac uptake of the radiotracer was observed in 80% of AL patients (n = 5) who received ≥ 1 mCi 124I-p5+14, with a mean myocardium:blood pool ratio of 2.3 ± 0.5 in positive images. Additionally, liver, spleen and kidney retention of 124I-p5+14 was observed in 20%, 40%, and 60% of AL patients, respectively. Furthermore, retention of the radiotracer has been indicated in the heart, spleen and kidney in patients with ATTR and ALECT2--associated amyloidoses.
Conclusion: Initial PET/CT image data indicate that 124I-p5+14 can provide quantitative detection of systemic AL amyloidosis in multiple organ systems and may have general utility in detecting and monitoring amyloid burden in many forms of amyloidosis.
Acknowledgments: This study was supported in part by the National Heart Lung and Blood Institute, National Institutes of Health, through the Science Moving TowArds Research Translation and Therapy (SMARTT) program via the following contracts: HHSN268201600011C, HHSN268201600012C, and HHSN268201600014C, as well as by contributions from Gerdau to the ACTP Gift Fund at the UTGSM and support from UHS. In addition, we thank Michael Stabin, PhD, Brett Hines, Carmella Moody, PhD and Derry Ridgway, MD and the many patients for their support.
Wall:Solex LLC: Equity Ownership; University of Tennessee Research Foundation: Patents & Royalties. Stuckey:Solex LLC: Equity Ownership. Martin:Solex LLC: Equity Ownership. Richey:Solex LLC: Equity Ownership. Ramchandren:Sandoz-Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Pharmacyclics LLC, an Abbvie company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Research Funding; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding. Kennel:University of Tennessee Research Foundation: Patents & Royalties; Solex LLC: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal