Introduction: The Canadian tyrosine kinase inhibitor (TKI) discontinuation trial has reported a 59.1% and 21.5% molecular relapse-free survival (RFS) rate after first attempt of treatment free remission (TFR1) with imatinib (IM) discontinuation and after a 2nd attempt of TFR (TFR2) with dasatinib (DA) discontinuation, respectively. Throughout the first and second attempts of TKI discontinuation, the kinetics of BCR-ABL qPCR transcript rise were very similar after TFR1 and TFR2. This prompts us to have a better understanding of the dynamics of BCR-ABL qPCR rise after TKI discontinuation.
Methods and materials: This prospective clinical trial (BMS CA180-543, Clinicaltrial.gov NCT#02268370) has 3 phases: 1) IM discontinuation phase, 2) DA rechallenge phase, 3) DA discontinuation phase. We have analyzed the monthly BCR-ABL1 qPCR value and doubling time (DT) in the first 6 months following IM discontinuation. The qPCR level before IM discontinuation or the qPCR level from the prior month was used as a baseline. DT at each measurement was calculated as x = ln(2)/K, where x is the DT and k is the fold BCR-ABL1 change from the previous value divided by the number of days between each measurement. The distribution of DT for all patients was assessed at each timepoint of DT measurement within the first 6 months.
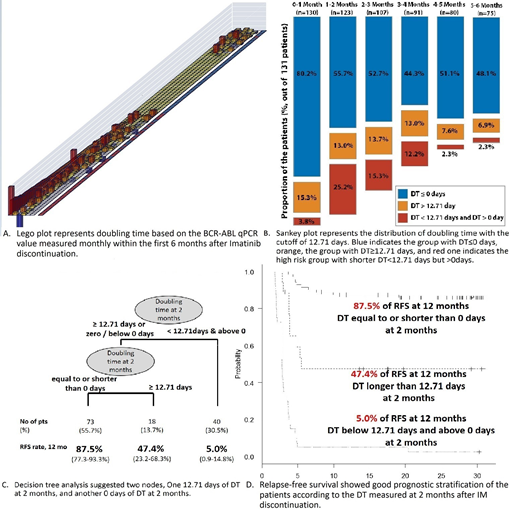
In order to define the best cut-off levels of BCR-ABL1 qPCR and DT showing the best risk stratification power throughout the first 6 months, DT values were collected and analyzed for molecular relapse-free survival (RFS) from the time of DT measurement. Then, a binary recursive partitioning method was applied using RFS which is calculated from the time of each DT measurement. Based on the DT cut-off value, the group was divided into 2 groups. The RFS was compared according to the groups.
Results: As of March 25, 2019, out of 131 patients enrolled, 58 patients (44.3%) lost a molecular response. The 6- and 12-months' molecular relapse-free survival (mRFS) rate was estimated as 59.1% (50.1-67.0%) and 56.8 % (47.8-64.8%), respectively.
BCR-ABL1 qPCR transcript level after IM discontinuation showed a rapid rise between the first 2-4 months, followed by a gradual rise after 4 months. The proportion of the patients showing DT less than 12.71 days but above 0 was 3.8% at 1 mo, 25.2% at 2 mo, 15.3% at 3 mo, 12.2% at 4 mo, 2.3% at 5 mo and 2.3% at 6 mo, respectively.
DT values were collected and analyzed for molecular RFS from the time of DT measurement. Binary recursive partitioning method was applied and provided 12.71 days as the best DT cutoff value to stratify the patients according to the RFS from the time of each DT measurement. In other words, the patients having DT less than 12.71 days but above 0 at any time within the first 6 months had a higher risk of failing the TFR attempt, while those with DT equal to or over 12.71 days at any time has a lower risk of losing TFR after IM discontinuation. The best result was reported in the group with stable BCR-ABL qPCR transcript level.
A rapid incline of BCR-ABL qPCR transcript level was observed 2-4 months after IM discontinuation. According to the DT measured at 2 months, the group with DT less than 12.71 days but above 0 showed the lowest mRFS rate of 5.0% (0.9-14.8%) at 12 months (HR 5.74), compared to the group with DT equal to/over 12.71 days (12 months' mRFS 47.4% [23.2-68.3%]) or the group with DT equal to/less than 0 days (12 months' mRFS 87.5% [77.3-93.3%]; p<0.001 [i.e. 3.5x10-32]).
Decision tree analysis was performed including 4 variables such as DT below 12.71 days, DT equal to or below 0 days, total IM treatment duration and MR4 response duration. The first node was DT below 12.71 days, and the second was DT equal to/less than 0 days. Total IM duration or MR4 duration were not identified as significant in the decision tree analysis.
Multivariate analysis confirmed that grouping based on the DT at 2 months is an independent risk factor for TFR. The group with DT below 12.71 but above 0 showed 5.74 times higher risk of losing TFR after IM discontinuation independent of the total duration of IM treatment.
Conclusion: DT with cut off value of 12.71 days at 2 months based on the BCR-ABL1 qPCR transcript level measured in the first 6 months after IM discontinuation is predictive of TFR failure after IM discontinuation.
Busque:ExCellThera: Patents & Royalties; BMS: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Paladin: Consultancy. Savoie:Pfizer: Consultancy; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Keating:Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Seattle Genetics: Consultancy; Janssen: Membership on an entity's Board of Directors or advisory committees; Shire: Membership on an entity's Board of Directors or advisory committees; Hoffman La Roche: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Delage:Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding. Liew:Novartis: Consultancy, Honoraria. Leber:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal