INTRODUCTION
Sorafenib, a multi-kinase tyrosine kinase inhibitor (TKI) targets FLT3 internal tandem duplication (FLT3/ITD) mutations and has efficacy in adult FLT3/ITD+ AML. High allelic ratio (HAR) FLT3/ITD mutations (allelic ratio of > 0.4, referred to as FLT3/ITD+) confer poor prognosis in de novo pediatric AML. COG AAML1031 Arm C studied the feasibility and efficacy of adding sorafenib to standard chemotherapy and as a single agent maintenance therapy in pediatric HAR FLT3/ITD+ patients.
METHODS
The AAML1031 primary randomization (Arm A vs. B) has been previously reported (Aplenc et al. Blood 2016;128:899). Patients with FLT3/ITD+ AML were offered secondary enrollment on Arm C and toxicities were monitored on respective treatment arms. An initial safety phase (Cohort 1, C1; N=12) defined the maximum tolerated dose (MTD) of sorafenib when added to induction II chemotherapy and subsequent courses. Following completion, the study was amended (Cohort 2; C2) to start sorafenib on day 11 of induction I and concomitantly with chemotherapy in subsequent cycles. A year of sorafenib maintenance was also added. Due to concerns for cardiac toxicity on interim analyses, the study was amended (Cohort 3; C3) to initiate sorafenib after chemotherapy completion in all courses. Sorafenib pharmacokinetics (PK) and plasma inhibitory activity (PIA) were measured in a subset of patients. Clinical outcome analysis was limited to cohorts 2/3 given lack of induction I sorafenib exposure in C1. Arm C results were compared to N=34 HAR FLT3/ITD+ AML patients on the control arm of COG AAML0531 (Arm A), a group that received comparable chemotherapy without sorafenib. Complete remission (CR), event-free survival (EFS), overall survival (OS) and relapse risk (RR) of patients with HAR FLT3/ITD AML enrolled on the 2 trials were compared to assess the impact of sorafenib use. Cox proportional hazards were used for multivariable analyses.
RESULTS
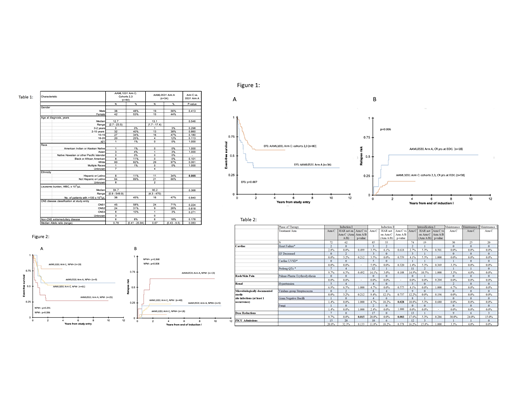
In the dose finding phase (C1), 12 patient were enrolled and the MTD of sorafenib defined as 200 mg/m2; dose limiting toxicities included rash (N=2; 1 grade III, 1 grade II), grade II hand foot syndrome and grade III fever. For the evaluation of efficacy, 80 C2/C3 (C2: N=33, C3: N=47) patients were enrolled. 30/80 (37.5%) received at least 1 maintenance cycle; 20/80 (25%) completed all courses. As outcomes were compared to N=34 HAR FLT3/ITD+ AML patients enrolled on the preceding trial AAML0531 Arm A as a historical comparison, clinical characteristics were described for the 2 groups (Table 1). For AAML1031 C2/C3, rates of induction I CR were 73% vs. 56% for AAML0531 Arm A (p=0.078). Following induction II, CR rates were 91% and 70% respectively (p=0.007). 3 year EFS from study entry was 57.5% for Arm C vs. 34.3% for AAML0531 Arm A; (p=0.007) whereas 3 year OS from study entry was not significantly different (63.9% vs. 54.1%, p=0.375, Figure 1A). RR from CR was reduced with sorafenib treatment (Arm C 18.2% vs. historical 52.5%, p=0.006, Figure 1B). The improvement in EFS and RR observed with sorafenib was retained in those with wild-type nucleophosmin (NPM-) FLT3/ITD+ AML but lacked statistical significance compared to historical in NPM+ FLT3/ITD+ disease (Figure 2A/2B). As 21/34 (62%) patients in the historical control did not undergo HSCT there was concern that the improved outcome observed for Arm C reflected increased use of HSCT in AAML1031. Multivariable analysis that adjusted for HSCT resulted in significantly lower EFS and higher RR for FLT3/ITD+ patients on AAML0531, suggesting that increased use of HSCT on Arm C did not explain the improved outcomes observed (EFS: HR 2.04, CI 1.18 - 3.53; p=0.01, RR: HR 3.09, CI 1.28 - 7.49; p=0.012). While dose modifications were more frequent for FLT3/ITD+ patients on Arm C versus arms A/B of AAML1031, targeted toxicity rates were otherwise no higher (Table 2). PIA assays demonstrated median trough FLT3 inhibition of 92% (n=29, 95% CI 79-94%), 91% (n=84, 95% CI 87-94%) and 81% (n=70, 95% CI 76-90%) for induction I, induction II and intensification I, respectively.
CONCLUSION
Addition of sorafenib to Arm C of AAML1031 was safe and resulted in potent FLT3 inhibition, particularly early in therapy. Sorafenib improved rates of induction II CR as well as 3 year EFS and reduced RR from CR compared to historical controls. These data support use of sorafenib in pediatric patients with HAR FLT3/ITD+ AML.
Fisher:Pfizer: Research Funding; Astellas: Other: Data Safety Monitoring Board Chair for an antifungal study; Merck: Research Funding. Levine:Viracor: Patents & Royalties: biomarker patent; Biogen: Other: non-financial support; Ironwood: Consultancy; Bluebird Bio: Consultancy; National Cancer Institute: Research Funding; Novartis: Consultancy; Incyte: Consultancy, Research Funding; Kamada: Research Funding. Loken:Hematologics, Inc: Employment, Equity Ownership.
sorafenib for high allelic ratio FLT3/ITD+ AML
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal