Introduction
Central nervous system lymphoma (CNSL) is a rare neoplasia and arises as primary (PCNSL) or secondary CNSL (SCNSL) and most commonly occurs as diffuse large B-cell lymphoma. Although prognosis of this disease has significantly improved due to advantages in diagnostic procedures and intensification of treatment over the last decade there remain major challenges in the clinical management. Magnetic resonance imaging (MRI) - as the standard imaging modality - has difficulties to discriminate CNSL from other brain-derived tumors or metastasis. Moreover, prognostic scores in CNSL lack the ability to reliable define patients with high relapse risk 1. Therefore, novel strategies to facilitate diagnosis and to select patients that profit from intense treatment protocols are urgently needed.
The C-X-C chemokine receptor 4 (CXCR4) is a transmembrane chemokine receptor with pivotal roles in cell homing and is often overexpressed in hematologic malignancies. CXCR4-directed positron emission tomography (PET) imaging with the tracer [68Ga]Pentixafor has been proven to be a suitable in vivo imaging modality for CXCR4 expression in lymphoid malignancies 2.
To evaluate the feasibility of CXCR4-directed PET and the prognostic value of this imaging modality this retrospective proof-of-concept study evaluated [68Ga]Pentixafor PET imaging in patients with CNSL.
Methods
11 patients with lymphoma of the CNS (n=8 PCNSL, n=3 SCNSL) were imaged with the CXCR4-directed PET tracer [68Ga]Pentixafor in this retrospective proof-of-concept study after signing inform consent. Lymphoma tissue was assessed for CXCR4 expression ex vivo by immunohistochemistry.
The prognostic value of CXCR4-directed PET imaging was evaluated in a computed analysis in 7 patients with follow-up MRI. Treatment response calculated as treatment efficiency η by sequential MRI was correlated with [68Ga]Pentixafor PET derived parameters at diagnosis such as volume of PET positive lymphoma lesion V(PET), maximal PET uptake value within the lesion max(PET) or integrated uptake values over the lymphoma lesion ∫(PET). Analysis was performed in a lesion- and patient-based manner.
Results
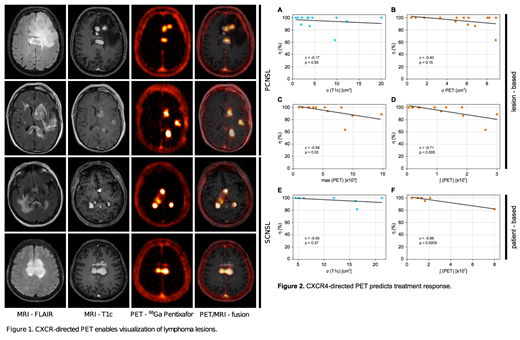
[68Ga]Pentixafor PET imaging was positive in all patients with active disease (10/11 patients) with excellent contrast characteristics to the surrounding brain parenchyma. PET positive lesions correlated well with lymphoma lesions in MRI-T1c sequences. The surrounding edema depicted in MRI-FLAIR sequences was evaluated as PET negative (Figure 1). Semi-quantitative analysis revealed maximum standard uptake values of [68Ga]Pentixafor-derived PET from 4.2 to 23.3 within the lesions with a high tumor-to-background-ratio ranging from 13.2 to 83.0.
The computed analysis revealed that CXCR4-directed PET parameters at diagnosis given by max(PET) and ∫(PET) significantly correlated with treatment response in the lesion-based as well as in the patient-based analysis. Specifically, ∫(PET) was the most significant prognostic factor in the present study (Figure 2 C, D, F). On the other hand tumor volume at diagnosis measured either by MRI or by [68Ga]Pentixafor PET did not denote a predictive marker for treatment response (Figure 2 A, B, E).
Conclusion/Outlook
CXCR4-directed PET imaging represents a novel imaging modality for CNSL. Due to its excellent contrast properties it might help to facilitate diagnosis and refine response assessment. Moreover, owing the predictive value for treatment response, CXCR4-directed PET could serve as a biomarker for the selection of patients profiting from intense treatment protocols. Furthermore, the feasibility of endoradiotherapeutic approaches targeting CXCR4 with Pentixather - the therapeutic twin of the imaging peptide Pentixafor - has been shown in hematologic malignancies and could be incorporated in the treatment of CNSL.
References
1. Han CH, Batchelor TT. Diagnosis and management of primary central nervous system lymphoma. Cancer. 2017;123(22):4314-4324.
2.Kircher M, Herhaus P, Schottelius M, et al. CXCR4-directed theranostics in oncology and inflammation. Ann Nucl Med. 2018;32(8):503-511.
Wester:Scintomics: Other: Spouse CEO of Company; CXCR4-targeted radiopharmaceuticals: Other: Inventor; Scintomics GmbH, Germany: Other: Shareholder. Bassermann:Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal