Background:
High-intermediate or high risk in international prognostic index (IPI) has a long-term chance of cure in the range about 50% in patients with diffuse large B cell lymphoma (DLBCL) treated by R-CHOP. These high risk patients should be considered for additional new treatment to standard R-CHOP or investigational approaches in the context of clinical trials that are designed to ensure that potentially curative therapy. Bortezomib inhibits NF-κB activation through proteasome inhibition, providing rationale for its use in cells that constitutively express NF-κB. Non-germinal center B cell (GCB) DLBCL has a worse survival after upfront chemotherapy and is characterized by constitutive activation of the antiapoptotic NF-κB pathway, which can inhibit chemotherapy. There is no study of bortezomib as maintenance therapy after treated with R-CHOP in high risk patients with DLBCL. So we applied additional bortezomib as maintenance therapy in order to assess improving efficacy and survival rates in high risk patients with non-GCB DLBCL who had been confirmed complete response (CR) after treated with R-CHOP.
Methods:
Patients with newly diagnosed stage II(bulky)-IV DLBCL with high or high intermediate IPI score of 3 to 5, and patients achieving a CR at the end of 6 or 8 cycles of R-CHOP21 were eligible for enrollment. Non-GCB DLBCL according to Hans criteria confirmed by central review was need before enrollment. Bortezomib maintenance treatment was consisted of bortezomib 1.3mg/m2 subcutaneously administration day 1 and day 15 per 28-day cycle with a total of 12 cycles. The primary endpoint was 3-year progression-free survival (PFS). Secondary endpoints were 3-year overall survival (OS), and toxicites. Toxicity was graded according to the Common Terminology Criteria for Adverse Events v4.0.
Results:
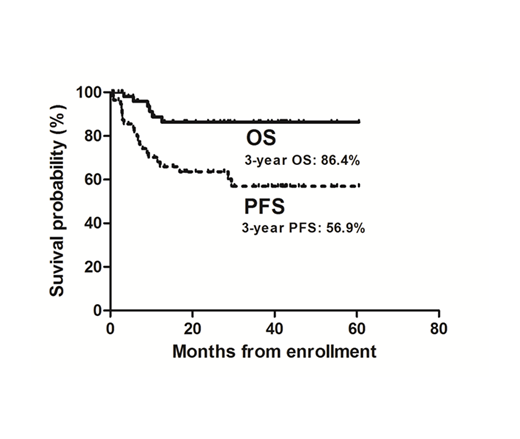
Fifty-nine patients were enrolled between May 2014 and Oct 2018. The type of Non-GCB DLBCL in all patients was confirmed by the central pathology review. The median age was 65 years (range: 27-86 years), and 60% were > 61 years. The baseline clinical features were as follows: female sex, 45.8%; ECOG >1, 10.2%; stage II bulky (>10cm), 6.8%; stage III/IV, 93.2%. At the time of analysis, 29 patients completed 12-cycles of bortezomib maintenance, and 3 patients is ongoing. Seven patients did not finished maintenance therapy due to toxicities (fatigue, atrial flutter, neuropathy, pleural effusion, thrombocytopenia), and withdrawal of informed consent (n=4). Sixteen patients experienced disease progression during bortezomib maintenance treatment. With a median follow-up of 25.1 months, 3-year PFS rate was 56.9% and 3-year OS rate was 86.4% (Figure 1). Toxicity was assessed in 489 cycles of bortezomib maintenance in all 59 patients. There was no treatment-related death and febrile neutropenia.
Conclusion:
Bortezomib maintenance showed 3-year PFS rate of 56.9% with acceptable toxicities in patients with high risk DLBCL achieving a CR at the end of 6 or 8 cycles of R-CHOP21.
Kim:Celltrion: Research Funding; Novartis: Research Funding; J + J: Research Funding; Donga: Research Funding; Kyowa-Kirin: Research Funding; Novartis: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal