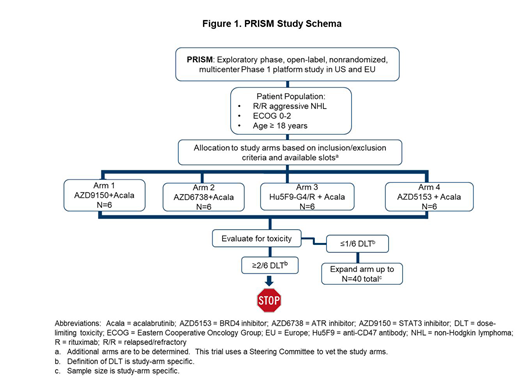
Aggressive B-cell lymphomas are genetically and clinically heterogeneous. Standard clinical trial designs do not efficiently evaluate the safety and efficacy of multiple drug combinations within the context of underlying molecular biology. The rapid identification of oncogenic driver pathways and development of multiple targeted drugs in lymphomas create many potential combinations. To address this need, we developed a Phase 1 master protocol termed PRISM (NCT03527147) to evaluate multiple targeted therapies alone or in combination for the treatment of relapsed/refractory (R/R) aggressive B-cell lymphoma. Each study arm is conducted in a predefined disease subset with the aim of addressing clinical and translational questions within an overarching protocol. All study arms are open label and not randomized. Enrolment of subjects into a given study arm is based on meeting inclusion/exclusion criteria and available slots. Pertinent inclusion criteria for the master protocol are: (a) a diagnosis of R/R non-Hodgkin lymphoma based on established World Health Organization criteria; (b) ≥1 prior line of therapy for the treatment of current histology, no known curative treatment options available, or the subject is ineligible for potential curative options; (c) the presence of radiographically measurable lymphadenopathy or extranodal lymphoid malignancy and; (d) an ECOG performance status ≤2. Exclusion criteria for the master protocol include: (a) a history of prior malignancy, severe or uncontrolled disease or conditions; (b) use of anti-lymphoma therapy within 14 days of the first dose of study drug and; (c) a requirement for ongoing immunosuppressive therapy. Treatment-specific inclusion/exclusion criteria are also provided (see www.clinicaltrials.gov).
As PRISM has multiple study arms, subjects can be simultaneously screened for multiple arms. In each arm, a safety review for dose-limiting toxicity (DLT) is performed after 6 subjects have completed the protocol-defined DLT window. Further enrolment will only proceed in that arm if ≤1 subject experiences a DLT (Figure 1). The sample size for each respective arm is determined based on prior clinical/experimental data on anticipated/clinically meaningful activity of each drug combination. This determines a minimally acceptable response and a desirable response. For each arm, a futility analysis occurs after approximately 10 sequentially enrolled subjects. An arm is considered futile if there is <10% probability for the overall response rate (ORR) to be above the desirable response. A final analysis after approximately 21 enrolled subjects will determine whether the treatment should be studied further. The primary criterion for success is set as having >80% chance for the response rate to be above the minimally acceptable response. The study endpoints include safety, ORR, duration of response, progression-free survival, overall survival, and standard pharmacokinetic parameters.
Exploratory analyses include in depth translational studies employing peripheral blood and tumor tissue collected at screening and during treatment. These investigations aim to discover predictive biomarkers, identify the molecular correlates of response based on known genetic subtypes, investigate pharmacodynamic and pathway changes and define the depth of response using assays for measurable residual disease (MRD). Exploratory translational endpoints may inform additional biomarker selection strategies for future arms of the PRISM study.
All study arms within PRISM to date have combined acalabrutinib, a highly selective BTK inhibitor, with additional targeted agents in subjects with R/R diffuse large B-cell lymphoma.
The mechanism of action of the drugs combined with acalabrutinib are as follows:
1. AZD9150 is a 16-nucleotide antisense oligonucleotide designed to target and down-regulate expression of human STAT3 mRNA; administered intravenously.
2. AZD6738 is an inhibitor of ATR; administered orally.
3. Hu5F9-G4 is an anti-CD47 antibody and rituximab is an anti-CD20 antibody; both administered intravenously.
4. AZD5153 is a BRD4 inhibitor; administered orally.
In summary, PRISM is a unique platform protocol designed to efficiently evaluate targeted agents in R/R aggressive B-cell lymphoma with an emphasis on comprehensive translational and molecular investigations.
Izumi:AstraZeneca: Equity Ownership; Acerta Pharma: Employment, Equity Ownership, Patents & Royalties: Acalabrutinib patents. Hamdy:AstraZeneca: Equity Ownership; Acerta Pharma: Employment, Equity Ownership, Patents & Royalties: Acalabrutinib patents. Arkenau:Acerta Pharma: Research Funding. de Vos:Portola Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy; Verastem: Consultancy. Reagan:Kite, A Gilead Company: Consultancy; Curis: Consultancy; Seattle Genetics: Research Funding. Zinzani:Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Portola: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; Immune Design: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Honoraria, Speakers Bureau. Davies:BioInvent: Research Funding; ADCT Therapeutics: Honoraria, Research Funding; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Research Funding; Karyopharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Research Funding; Pfizer: Honoraria, Research Funding; Acerta Pharma: Honoraria, Research Funding; MorphoSys AG: Honoraria, Membership on an entity's Board of Directors or advisory committees. Pagel:AstraZeneca: Consultancy; Pharmacyclics, Inc.: Consultancy. Vose:Legend Pharmaceuticals: Honoraria; Acerta Pharma: Honoraria, Other: Grants, Research Funding; Bristol-Meyers Squibb Company: Research Funding; Celgene Corporation: Research Funding; Incyte Corporation: Research Funding; Kite Pharma: Honoraria, Other: Grants, Research Funding; Novartis: Research Funding; Seattle Genetics: Research Funding; AbbVie: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria. Bitman:Acerta Pharma: Employment; AstraZeneca: Equity Ownership. Brock:Acerta Pharma: Employment; AstraZeneca: Equity Ownership. Clark:AstraZeneca: Employment, Equity Ownership. Frigault:Acerta Pharma: Employment; AstraZeneca: Employment, Equity Ownership. Ware:Acerta Pharma: Employment; Astrazeneca: Employment, Equity Ownership. Yang:Acerta Pharma: Employment; AstraZeneca: Equity Ownership. Staudt:Nanostring: Patents & Royalties. Flinn:TG Therapeutics, Trillum Therapeutics, Abbvie, ArQule, BeiGene, Curis, FORMA Therapeutics, Forty Seven, Merck, Pfizer, Takeda, Teva, Verastem, Gilead Sciences, Astra Zeneca (AZ), Juno Therapeutics, UnumTherapeutics, MorphoSys, AG: Research Funding; Acerta Pharma, Agios, Calithera Biosciences, Celgene, Constellation Pharmaceuticals, Genentech, Gilead Sciences, Incyte, Infinity Pharmaceuticals, Janssen, Karyopharm Therapeutics, Kite Pharma, Novartis, Pharmacyclics, Portola Pharmaceuticals: Research Funding; AbbVie, Seattle Genetics, TG Therapeutics, Verastem: Consultancy; TG Therapeutics, Trillum Therapeutics, Abbvie, ArQule, BeiGene, Curis, FORMA Therapeutics, Forty Seven, Merck, Pfizer, Takeda, Teva, Verastem, Gilead Sciences, Astra Zeneca (AZ), Juno Therapeutics, UnumTherapeutics, MorphoSys, AG: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding.
acalabrutinib in DLBCL
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal