Introduction: T cell lymphomas are heterogenous with an overall poor prognosis. Peripheral T cell lymphoma not otherwise specified (PTCL-NOS) and angioimmunoblastic T cell lymphoma (AITL) are the two most common subtypes in the US accounting for 45% of diagnoses. Based on overlapping recurrent molecular and cytogenetic abnormalities, the WHO created an umbrella category of nodal T-cell lymphomas with T-follicular helper phenotype (TFH lymphoma), which includes AITL and PTCL with TFH phenotype (Swerdlow SH, et al. Blood. 2016). 25-30% of patients (pts) are refractory to initial therapy and even amongst responders to initial therapy, relapse is common. Survival after relapse or progression (R/P) is typically measured only in months. Outcomes have not changed significantly even with the introduction of several new agents in the last 10 years (Chihara D, et al. Br J Haematol. 2017). There remains no standard 2nd line therapy or guidance on sequencing of therapies as interpretation of clinical data in T-cell lymphoma is frequently hampered by the heterogeneity of the patient population and small sample size. The aim of our study was to determine outcomes in a large, well-defined group of pts with either primary refractory PTCL-NOS or TFH lymphoma.
Methods: We performed a multi-center retrospective study to determine outcomes to 2nd line therapy for adult pts diagnosed between 1.1.09-6.30.18 with PTCL-NOS or TFH lymphoma, who were primary refractory defined by either induction failure, less than CR to initial therapy, or relapse within 6 months (mo) of completion of initial therapy. We performed time to event analysis using Kaplan-Meier method and compared groups using log-rank test. All other statistics were descriptive.
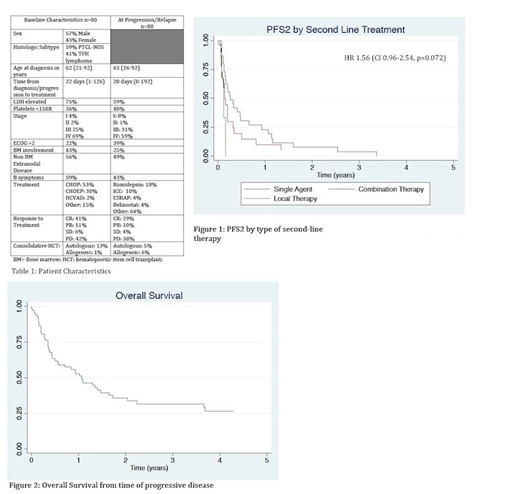
Results: Patient and disease characteristics at diagnosis and relapse are summarized in Table 1. We identified 80 eligible pts from 7 US academic centers. Median FU was 17.2 mo from diagnosis (0.5-70). Overall, pts had mostly advanced stage with multiple high-risk features including bone marrow (BM) or extranodal (EN) involvement and B symptoms at diagnosis and R/P. Most pts received CHOP (52%) or CHOEP (24%) as initial therapy. 60% of patients did not attain a CR, while 40% of pts relapsed after initial CR.14% had received a consolidative transplant in 1st CR prior to relapse (almost all autologous hematopoietic cell transplant [HCT]). At R/P, 48% received single agent therapy [mostly romidepsin (15/38)], while 36% received multi-agent salvage therapy [mostly ICE (9/29)]. 13/80 pts were placed on hospice or only received local therapy after R/P. Median OS from diagnosis and following R/P was 19 mo/12.9 mo respectively. Median PFS2 (defined as time from 2nd line therapy to 2nd progression) for pts receiving systemic therapy was 73 days (d) (range 41-175). On univariate and multivariate analysis, there was no significant difference in PFS2 by histologic subtype (74 d for PTCL and 73 d for TFH lymphoma; p=0.29), platelet count <150,000 (75 d vs 59 d; p=0.37), presence of B symptoms at R/P (78 d vs. 63 d, p=.16), ECOG PS ≥2 (75 d vs 57 d, p=0.62), elevated LDH (63 d vs 75 d, p=0.31) and EN disease at R/P (59 d vs 75 d, p=0.72). There was a trend towards longer PFS2 with single agent salvage therapy compared to combination salvage therapy (97 d vs. 51 d; p=0.09). There was no statistically significant difference in survival if pts had relapsed disease or induction failure (75 d vs. 50 d, p=0.97). Although numerically longer, OS did not differ statistically if pts underwent HCT as consolidation after 2nd line therapy (allogeneic HCT 29.1 mo, autologous HCT 48.5 mo vs. 16.1mo for no HCT; p=0.61, n=8). Median number of therapies after salvage was 1 (range 1-6).
Conclusions: Outcomes in this large, well-defined population of primary refractory PTCL-NOS and TFH lymphoma were poor, but better compared to most other retrospective series in R/R T-cell lymphoma. EN disease and advanced stage were common in this cohort of primary refractory pts. Relapse after HCT in CR1 had a particularly poor prognosis. Our findings suggest that single agent therapy following R/P in primary refractory pts and transplant may be beneficial, though our statistical power was limited due to small sample size. In a next step, we plan to expand the cohort and perform genetic/molecular characterization of available biospecimens following central pathology review.
Rhodes:DAVA Oncology: Honoraria. Olszewski:Spectrum Pharmaceuticals: Research Funding; Genentech: Research Funding; Adaptive Biotechnologies: Research Funding; TG Therapeutics: Research Funding. Brammer:Verastem, Inc: Research Funding; Viracta Therapeutics, Inc.: Research Funding; Bioniz Therapeutics, Inc.: Research Funding. Ghosh:TG Therapeutics: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Forty Seven Inc: Research Funding; AstraZeneca: Honoraria, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Genentech: Research Funding; SGN: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau. Dwivedy Nasta:Merck: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; 47 (Forty Seven): Research Funding; Rafael: Research Funding; Celgene: Honoraria; ATARA: Research Funding; Aileron: Research Funding; Debiopharm: Research Funding; Millenium/Takeda: Research Funding; Pharmacyclics: Research Funding. Barta:Celgene: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Honoraria; Seattle Genetics: Honoraria, Research Funding; Takeda: Research Funding; Celgene: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Research Funding; Mundipharma: Honoraria; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal