Background:
The acute and lymphoma subtypes Human T-lymphotropic virus 1-associated adult T-cell leukemia/lymphoma (HTLV-1 ATLL) are frequently characterized by chemo-refractoriness, a generally aggressive clinical course, and poor outcomes. Despite progress in characterizing the disease biology and the development and implementation of newer agents such as mogamulizumab, survival remains poor, especially for patients with relapsed or refractory disease. Furthermore, besides these many challenges, there is a paucity of comprehensive published data in Western (i.e. non-Japanese) patients.
Methods:
In a retrospective analysis, we identified 109 patients with pathologically-confirmed ATLL evaluated at our institution since 2001. 11 were excluded due to lack of clinical follow-up. Patient, disease, and treatment information was extracted for analysis. The International Prognostic Index (IPI) and Prognostic Index for PTCL-U (PIT) were calculated at time of diagnosis. Response rate was calculated based on the best response (CR/PR) during induction, with response retrospectively assessed based on available data using Lugano criteria. Median survival was calculated using Kaplan-Meier analysis and follow-up using a reverse Kaplan-Meier method, with survival and follow-up as of July 2019
Results:
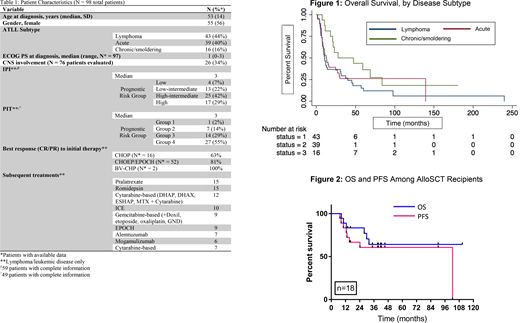
Ninety eight patients were included in our cohort (Table 1); 46 patients (47%) received initial treatment at another institution. The most common ATLL subtypes were lymphoma (n=43, 44%) acute (n=39, 40%), and smoldering/chronic (n=16, 16%). The median age at diagnosis was 53 years (range 30-92) and the median duration of follow-up from diagnosis was 65 months (95% CI: 41 to 178). With a median follow-up in survivors of 41.2 months, 21 patients are alive. The most common cause of death was disease (69/77 patients, 90%). The median overall survival (OS) among all patients was 13.2 months (Figure 1; range 1.3-240).
For acute/lymphoma subtype disease, most patients initially received EPOCH/CHOEP (54 patients), CHOP (18 patients), or BV-CHP (3 patients); practice patterns evolved since the late 2000's to include more etoposide and BV-CHP rather than CHOP. Among 76 patients with lymphoma/acute with reviewable restaging information, 30 (40%) achieved CR. For patients with active disease following induction, a variety of subsequent therapies were used, most commonly romidepsin and pralatrexate, each utilized in 15 patients.
Of 68 patients considered for transplant (55 saw a transplant physician, 13 had HLA typing sent), 23 patients ultimately underwent transplant, including 5 autologous and 18 alloSCT. Among patients referred who did not undergo transplant (N = 30), the most common reason was disease-related (24 patients, 80%) followed by patient preference (4 patients, 13%). Since 2010, only two patients underwent autoSCT at our center: one experienced primary graft failure necessitating autologous reconstitution and another lacked suitable allograft donor.
Most patients, 13/18 (72%), were in CR at time of alloSCT; 11 patients underwent transplant in first remission, 7 at subsequent timepoints. Donor and graft sources included matched related (n=6), one identical twin, matched unrelated (n=2), mismatched related (n=1), mismatched unrelated (n=2), cord blood (n=4) and haploidentical (n=2). Four patients relapsed after autoSCT, 6 patients following alloSCT, and 2 treatment-related deaths occurred post alloSCT. Progression-free and OS at median follow-up of 39.2 months among alloSCT recipients was 60.6% and 64.1%, respectively (Figure 2).
Conclusions:
We describe the treatment patterns and outcomes for a large series of non-Japanese patients at our center with ATLL. We confirm the poor outcomes with conventional therapy seen in other studies, with only 40% of lymphoma/leukemia subtype patients achieving CR with first-line therapy, and a median OS of 13.2 months among all patients. AlloSCT offers promise as an effective option that achieved durable long-term responses in many patients, yet its applicability is limited by chemorefractory disease. Larger studies are needed to confirm our findings and to identify effective salvage therapies to attain remission and bridge patients to alloSCT. For patients ineligible for alloSCT due to age/comorbidity, lack of suitable donor, or disease refractoriness, novel therapeutic approaches are urgently needed to improve outcomes.
Moskowitz:Bristol-Myers Squibb: Consultancy, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Erytech Pharma: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; Merck: Research Funding; Takeda Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Incyte: Research Funding; Merck: Research Funding; Erytech Pharma: Consultancy; ADC Therapeutics: Consultancy; Erytech Pharma: Consultancy; Merck: Research Funding; ADC Therapeutics: Consultancy; miRagen Therapeutics Inc: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; ADC Therapeutics: Consultancy; Erytech Pharma: Consultancy; Erytech Pharma: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Erytech Pharma: Consultancy; Erytech Pharma: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Incyte: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Cell Medica: Consultancy; Cell Medica: Consultancy; Cell Medica: Consultancy; Incyte: Research Funding; Takeda Pharmaceuticals: Consultancy; Cell Medica: Consultancy; Cell Medica: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Cell Medica: Consultancy; Incyte: Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; Merck: Research Funding; ADC Therapeutics: Consultancy; Merck: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; Takeda Pharmaceuticals: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Cell Medica: Consultancy; Takeda Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Merck: Research Funding; ADC Therapeutics: Consultancy; miRagen Therapeutics Inc: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Incyte: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Cell Medica: Consultancy; Incyte: Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Cell Medica: Consultancy; Cell Medica: Consultancy; Incyte: Research Funding; Takeda Pharmaceuticals: Consultancy; Incyte: Research Funding; Takeda Pharmaceuticals: Consultancy; Cell Medica: Consultancy; Cell Medica: Consultancy; Cell Medica: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Takeda Pharmaceuticals: Consultancy; Merck: Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Erytech Pharma: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Incyte: Research Funding; Incyte: Research Funding; Takeda Pharmaceuticals: Consultancy; Merck: Research Funding; ADC Therapeutics: Consultancy; Erytech Pharma: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Cell Medica: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Cell Medica: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Incyte: Research Funding; Incyte: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Incyte: Research Funding; Takeda Pharmaceuticals: Consultancy; Incyte: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; miRagen Therapeutics Inc: Consultancy, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Merck: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Takeda Pharmaceuticals: Consultancy; Merck: Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; ADC Therapeutics: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Takeda Pharmaceuticals: Consultancy; Erytech Pharma: Consultancy; Erytech Pharma: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Consultancy; miRagen Therapeutics Inc: Consultancy, Research Funding; Erytech Pharma: Consultancy; Erytech Pharma: Consultancy; Merck: Research Funding; ADC Therapeutics: Consultancy; ADC Therapeutics: Consultancy; ADC Therapeutics: Consultancy; ADC Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Erytech Pharma: Consultancy; Merck: Research Funding; Merck: Research Funding; Erytech Pharma: Consultancy; Kyowa Hakko Kirin Pharma: Consultancy, Research Funding; Merck: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding. Dogan:Roche: Consultancy, Research Funding; Corvus Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy; Celgene: Consultancy; Takeda: Consultancy; Novartis: Consultancy. Kumar:Seattle Genetics: Research Funding. Matasar:Genentech, Inc.: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding; Bayer: Consultancy, Honoraria, Other; Roche: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding; Merck: Consultancy, Equity Ownership; Juno Therapeutics: Consultancy; Teva: Consultancy; Rocket Medical: Consultancy, Research Funding; Seattle Genetics: Consultancy, Honoraria, Other: Travel, accomodation, expenses, Research Funding; Daiichi Sankyo: Consultancy; GlaxoSmithKline: Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Bayer: Other: Travel, accommodation, expenses. Noy:Medscape: Honoraria; Janssen: Consultancy; Prime Oncology: Honoraria; NIH: Research Funding; Pharamcyclics: Research Funding; Raphael Pharma: Research Funding. Perales:Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees; NexImmune: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; MolMed: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria; Medigene: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Kyte/Gilead: Research Funding; Miltenyi: Research Funding. Straus:Elsevier (PracticeUpdate): Consultancy, Honoraria; Hope Funds for Cancer Research: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Honoraria. Zelenetz:Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics/AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics/AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech/Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sauter:Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding; Spectrum Pharmaceuticals: Consultancy; Novartis: Consultancy; Genmab: Consultancy; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy; Celgene: Consultancy; GSK: Consultancy. Horwitz:Millennium/Takeda: Consultancy, Research Funding; Affimed: Consultancy; Celgene: Consultancy, Research Funding; Kura: Consultancy; Trillium: Research Funding; Mundipharma: Consultancy; Celgene: Consultancy, Research Funding; Kyowa Hakko Kirin: Consultancy; Portola: Consultancy; Kura: Consultancy; Infinity/Verastem: Consultancy, Research Funding; ADCT Therapeutics: Research Funding; Portola: Consultancy; Celgene: Consultancy, Research Funding; Portola: Consultancy; Astex: Consultancy; Seattle Genetics: Consultancy, Research Funding; Miragen: Consultancy; Miragen: Consultancy; Seattle Genetics: Consultancy, Research Funding; Aileron: Research Funding; ADCT Therapeutics: Research Funding; Forty-Seven: Research Funding; Aileron: Research Funding; Infinity/Verastem: Consultancy, Research Funding; Forty-Seven: Research Funding; Trillium: Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Innate Pharma: Consultancy; Astex: Consultancy; Celgene: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; Kura: Consultancy; Kyowa Hakko Kirin: Consultancy; Forty-Seven: Research Funding; Astex: Consultancy; Mundipharma: Consultancy; Innate Pharma: Consultancy; Trillium: Research Funding; Astex: Consultancy; Kyowa Hakko Kirin: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Millennium/Takeda: Consultancy, Research Funding; Affimed: Consultancy; Seattle Genetics: Consultancy, Research Funding; Aileron: Research Funding; Kyowa Hakko Kirin: Consultancy; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kura: Consultancy; Innate Pharma: Consultancy; Aileron: Research Funding; Innate Pharma: Consultancy; Mundipharma: Consultancy; Trillium: Research Funding; Affimed: Consultancy; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADCT Therapeutics: Research Funding; ADCT Therapeutics: Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium/Takeda: Consultancy, Research Funding; Miragen: Consultancy; Seattle Genetics: Consultancy, Research Funding; Mundipharma: Consultancy; Portola: Consultancy; Forty-Seven: Research Funding; Miragen: Consultancy; Infinity/Verastem: Consultancy, Research Funding; Affimed: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal