Introduction:Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is an indolent subtype of ALCL, arising in the space between the implant and the capsule. BIA-ALCL seems to be directly related to the exposure to breast implants, and usually happens more than 7 years from implant placement. In 2011 the Food and Drug administration released the first safety communication on the risk of BIA-ALCL for women with breast implants, raising awareness of the disease.
In 60-70% of the cases BIA-ALCL presents as a delayed seroma, a non-resolving fluid collection around breast implants arising after at least 1 year from implant placement. In previous studies, the risk of developing a late seroma ranged between 0.05% to 0.1% for people with textured surface breast implants. Among those with delayed seromas, the risk of BIA-ALCL has been estimated from retrospective cohorts to be 10%. (McGuire 2017, Clemens 2017, Maxwell 2015, Di Napoli 2017).
Methods:A prospective cohort study was conducted in the population that underwent breast reconstruction with textured breast implants after mastectomy for breast cancer by a single surgeon at Memorial Sloan Kettering Cancer Center (MSKCC). Patients were enrolled from December 1992 to December 2017 at the time of tissue expander insertion, underwent long-term follow up and major events related to implants were prospectively recorded. We actively started looking for BIA-ALCL in late seromas in early 2011. All cases of BIA-ALCL were analyzed by our hematopathology service at MSKCC. We identified all late seromas sent to hematopathology to exclude BIA-ALCL by cross checking all the pathology reports containing the words "lymphoma" or "anaplastic" or "ALCL" in this population. We calculated the cumulative incidence of clinically relevant late seromas, considering death as a competing risk with the statistical program R version 3.5.1.
Results:Between 1992 and 2017, 3521 women underwent breast reconstruction with textured surface expanders followed by permanent textured breast implants. Median age at surgery was 48 years (range 18-88), 1056 (30.0%) underwent unilateral reconstruction and 2464 (70.0%) bilateral reconstruction. Reason for mastectomy was lobular carcinoma in 675 (19.1%), 116 in situ, ductal carcinoma in 2529 (71.8%), 591 in situ, other histology in 59 (1.7%), unknown in 257 (7.3%). Besides surgery, additional treatments for breast cancer were: chemotherapy in 1015 (28.8%), radiotherapy in 285 (8.1%), chemo and radiotherapy in 706 (20.0%), none in 1514 (43.0%). Median follow up of the cohort was 8.2 years (range 3.4 months - 26.4 years).
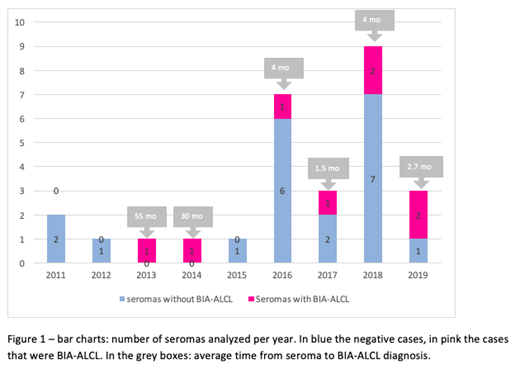
After a median exposure of 9 years (range 1.8 - 15.8 years), 28 women developed a clinically relevant delayed seroma, and 8 were diagnosed with BIA-ALCL (28.5% of the late seromas). The first late seroma was analyzed with flow cytometry in February 2011. All seromas sent to pathology were clinically relevant (main symptom was swelling of the breast - in 15 patients, tenderness/fullness of the breast - in 5 patients, asymmetry - in 3 patients, capsule contracture - in 5 patients). Three patients had previous drainage of peri-implant fluid that was not analyzed in pathology. 2 additional cases of BIA-ALCL were diagnosed in this population, one on an internal mammary chain lymph node biopsy, without fluid collection, and another with a capsular mass and lymph node involvement.
Rate of late seroma accounting for the varying follow-up and death as a competing event was 1.6% at 15 years. Rough percentage getting a seroma was 0.79%, 1/126 women exposed. All the 28 samples were analyzed with cytology, and 22/28 with flow cytometry including CD30. 14/28 (50%) of these women removed the implants for several reasons, 3/28 (10.7%) immediately exchanged the implants with another textured surface device.
Conclusions:in this population of breast cancer patients receiving textured breast implant reconstructions, the incidence of clinically relevant seromas and BIA-ALCL seems higher than previously reported. Similarly, the ratio of patients testing positive for BIA-ALCL on the total number of seromas analyzed higher than previously estimated. We believe these results will be informative for people with breast reconstructions after breast cancer and their surgeons, to better estimate the risk of seroma and BIA-ALCL with textured implants.
Cordeiro:Inamed: Research Funding; Acelity: Research Funding; Allergan: Research Funding. Dogan:Takeda: Consultancy; Novartis: Consultancy; Celgene: Consultancy; Seattle Genetics: Consultancy; Corvus Pharmaceuticals: Consultancy; Roche: Consultancy, Research Funding. Horwitz:Seattle Genetics: Consultancy, Research Funding; Millennium/Takeda: Consultancy, Research Funding; Trillium: Research Funding; Kyowa Hakko Kirin: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Kyowa Hakko Kirin: Consultancy; Astex: Consultancy; Portola: Consultancy; Affimed: Consultancy; Infinity/Verastem: Consultancy, Research Funding; Kyowa Hakko Kirin: Consultancy; Kura: Consultancy; Celgene: Consultancy, Research Funding; Affimed: Consultancy; Trillium: Research Funding; Forty-Seven: Research Funding; Miragen: Consultancy; Mundipharma: Consultancy; Aileron: Research Funding; Affimed: Consultancy; ADCT Therapeutics: Research Funding; Trillium: Research Funding; ADCT Therapeutics: Research Funding; Innate Pharma: Consultancy; Affimed: Consultancy; Forty-Seven: Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Portola: Consultancy; Portola: Consultancy; Infinity/Verastem: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Kyowa Hakko Kirin: Consultancy; Mundipharma: Consultancy; Mundipharma: Consultancy; Infinity/Verastem: Consultancy, Research Funding; Innate Pharma: Consultancy; Aileron: Research Funding; Innate Pharma: Consultancy; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Miragen: Consultancy; Miragen: Consultancy; Corvus Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mundipharma: Consultancy; Kura: Consultancy; Kura: Consultancy; Aileron: Research Funding; ADCT Therapeutics: Research Funding; Innate Pharma: Consultancy; Astex: Consultancy; ADCT Therapeutics: Research Funding; Astex: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Miragen: Consultancy; Astex: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Forty-Seven: Research Funding; Infinity/Verastem: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Aileron: Research Funding; Kura: Consultancy; Trillium: Research Funding; Portola: Consultancy; Forty-Seven: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal