Introduction: For patients (pts) with relapsed indolent non-Hodgkin lymphoma (iNHL) who develop resistance to rituximab, treatment options are limited and the prognosis is poor. The open-label, randomized, Phase III GADOLIN (NCT01059630) study compared the efficacy and safety of obinutuzumab (GA101; G) plus bendamustine (B) induction, followed by G maintenance (G-B arm), with B induction (B arm; standard of care) in rituximab-refractory iNHL. The primary analysis in 396 pts (data cutoff: September 1, 2014; median observation time, 21.0 months) showed that Independent Review Committee (IRC)-assessed progression-free survival (PFS; primary endpoint) was significantly longer with G-B (median not reached [NR]) vs B (14.9 months), corresponding to a 45% reduction in risk of progression or death (hazard ratio [HR], 0.55; 95% confidence interval [CI]: 0.40, 0.74; p=0.0001; Sehn et al. Lancet Oncol 2016). The safety profile of G-B was manageable. Here, we report the final analysis of efficacy and safety for GADOLIN (when safety follow-up for all pts had been completed [2 years' safety follow-up from last dose]; data cutoff: November 30, 2018).
Methods: Enrolled pts were aged ≥18 years with documented rituximab-refractory iNHL and an ECOG performance status of 0-2. Pts received either G 1000mg intravenously (i.v.) (Days [D] 1, 8, and 15 of Cycle [C] 1, and D1 of C2-6) plus B 90mg/m2/day i.v. (D1 and 2 of C1-6) or B monotherapy (120mg/m2/day i.v., D1 and 2 of C1-6) in 28-day cycles. Following induction, pts in the G-B arm without evidence of progression received G maintenance (1000mg i.v. every 2 months for 2 years or until disease progression). Final analysis endpoints included investigator (INV)-assessed PFS, overall survival (OS), time to new anti-lymphoma treatment (TTNT), and safety. The safety population included pts who received ≥1 dose of study treatment, excluding two pts crossing over to G-B during maintenance.
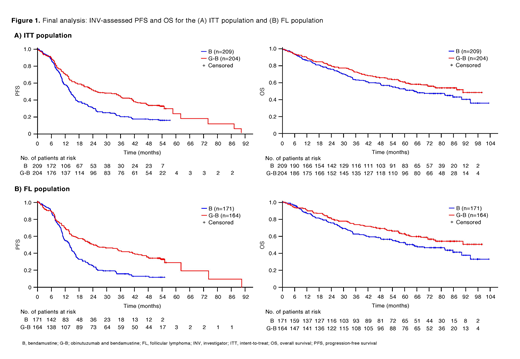
Results: Of 413 iNHL pts in the ITT population (G-B, 204; B, 209), 335 (G-B, 164; B, 171) had follicular lymphoma (FL). Median (range) observation time was 57.5 (0.4-97.6) months for the G-B arm and 47.9 (0-100.9) months for the B arm (i.e. 27.6 and 35.6 months additional follow-up since the primary analysis). Median INV-assessed PFS was 25.8 months for the G-B arm vs 14.1 months for the B arm (HR, 0.57; 95% CI: 0.45, 0.73; p<0.0001) in all iNHL pts (Figure 1A). Overall, fewer iNHL pts died in the G-B (84/204; 41.2%) than in the B (100/203; 49.3%) arm; median OS was 88.3 vs 65.6 months (HR, 0.77; 95% CI: 0.57, 1.03; p=0.0810; 23% risk reduction [Figure 1A]). Median TTNT was also longer with G-B vs B (38.2 vs 18.9 months, respectively [HR, 0.60; 95% CI: 0.47, 0.76]). Results for FL pts were as follows: median PFS, 24.1 vs 13.7 months (HR, 0.51; 95% CI: 0.39, 0.67; p<0.0001 [Figure 1B]); median OS, NR vs 60.3 months (HR, 0.71; 95% CI: 0.51, 0.98; p=0.0343); median TTNT, 33.6 vs 18.0 months (HR, 0.56; 95% CI: 0.43, 0.73). In the safety population (N=407; G-B, 204; B, 203), 149/204 (73.0%) of pts in the G-B arm and 134/203 (66.0%) in the B arm experienced grade ≥3 adverse events (AEs). Compared with B, grade ≥3 neutropenia (37.3% vs 30.0%) and grade ≥3 infusion-related reactions (11.3% vs 5.4%) were more frequent with G-B, and grade ≥3 thrombocytopenia (10.8% vs 15.8%) and anemia (7.4% vs 10.8%) were less frequent. The incidences of grade ≥3 infections (22.5% vs 19.2%) and grade ≥3 second malignancies (7.8% vs 5.9%) were similar between arms. The proportion of pts with serious AEs was 91/204 (44.6%) in the G-B arm and 76/203 (37.4%) in the B arm; fatal AEs were reported in 20/204 (9.8%) and 15/203 (7.4%) of pts, respectively. The most frequent AEs leading to death in the G-B vs B arms were infections (six vs seven pts, respectively) and second malignancies (eight vs four pts, respectively). Safety results in FL pts were comparable with all iNHL pts.
Conclusions: Final analysis of the GADOLIN study showed that G-B was associated with a 43% reduction in the risk of progression or death compared with B in pts with rituximab-refractory iNHL and a 49% reduction in pts with FL, with a sustained and clinically relevant OS benefit in pts with FL. There were no new safety signals with longer follow-up.
Acknowledgments: The GADOLIN study was sponsored by F. Hoffmann-La Roche Ltd. Third party medical writing assistance, under the direction of Laurie Sehn, was provided by Louise Profit of Gardiner-Caldwell Communications and was funded by F. Hoffmann-La Roche Ltd.
Sehn:TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Apobiologix: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Verastem: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; Janssen-Ortho: Consultancy, Honoraria; Janssen-Ortho: Honoraria; Celgene: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Merck: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; Astra Zeneca: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; Merck: Consultancy, Honoraria. Trněný:Takeda: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Celgene: Consultancy; Gilead Sciences: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; F. Hoffmann-La Roche: Consultancy, Honoraria. Bouabdallah:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Dueck:Amgen: Consultancy; Celgene: Consultancy; Janssen: Consultancy; Roche: Research Funding. Gribben:Abbvie: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Acerta/Astra Zeneca: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Lugtenburg:Celgene: Honoraria; Janssen-Cilag: Honoraria; Servier: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Roche: Honoraria, Research Funding, Speakers Bureau; Genmab: Honoraria. Salles:Epizyme: Consultancy, Honoraria; BMS: Honoraria; Roche, Janssen, Gilead, Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis, Servier, AbbVie, Karyopharm, Kite, MorphoSys: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events; Autolus: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Other: Educational events; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events. Knapp:F. Hoffmann-La Roche Ltd: Employment. Nielsen:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Liu:Roche Pharma Development, Shanghai, China: Employment. Cheson:Seattle Genetics: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trillium: Research Funding; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Symbios: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Research Funding; Portola: Research Funding; Kite: Research Funding; Gilead: Research Funding; Epizyme: Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Acerta: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal