Introduction
Acute myeloid leukemia (AML) is categorized into favorable-, intermediate- and adverse-risk groups according to European LeukemiaNet (ELN) risk stratification. The intermediate-risk AML comprises a substantial proportion of AML, and even within the intermediate-risk AML, the biology and prognosis are highly different, which suggests that more prognostic factors are needed to be identified.
Method
A total of 265 newly diagnosed AML patients treated between January 2010 and January 2019 who had cryopreserved DNA for mutational analyses diagnosed and treated in Changhai Hospital were included. 121 patients were aged ≤ 65y, and classified as intermediate-risk (IR)-AML. A Custom Amplicon panel targeting exons of 210 genes was used for deep sequencing at diagnosis. We used a LASSO Cox regression model to achieve shrinkage and variable selection from prognostic factors (P<0.1 in log-rank tests) to build risk score for predicting overall survival. A nomogram was constructed to display the risk of death in individuals. The discrimination of the risk score was measured by the concordance index (C-index) and areas under time-dependent receiver-operating characteristics (ROC) curves (AUCs), and the calibration of the risk score was explored graphically by calibration plots. Patients with IR-AML and aged ≤ 65y in The Cancer Genome Atlas (TCGA) data (n= 41) was used as a validation cohort.
Results
The median age was 44 (11-75) years, and 54% had normal karyotype. NRAS and CEBPA were the most recurrently mutated genes (both 26%), followed by KIT (24%), DNMT3A (23%), ARID1B (20%), FLT3-ITD (19%), PCLO (17%), and TET2 (17%).
In univariate analyses, age ≥ 55 years, WBC ≥ 10×109/L , PLT > 40×109/L, high LDH counts at diagnosis, DNMT3A and Signaling Pathway genes mutations and the number of mutated genes ≥ 8 were significantly associated with poor OS (P < 0.05), and age ≥ 55 years, WBC≥ 10×109/L, DNMT3A and FLT3-ITD mutations were associated with worse relapse free survival(RFS, P < 0.05).
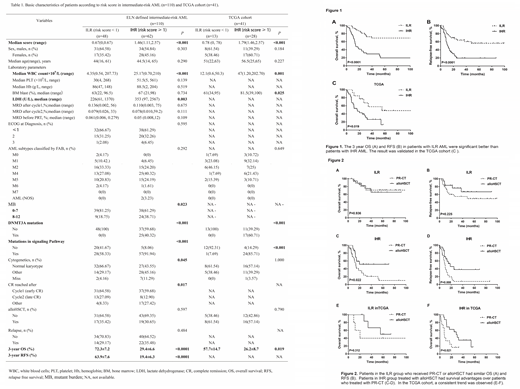
Three variables were incorporated in our scoring model by LASSO, including Signaling Pathway genes mutations (including NRAS, KIT, FLT3, KRAS etc), DNMT3A mutation and WBC≥ 10×109/L. A risk scoring model was developed incorporating the weighted coefficients of these variables:0.6749 × Signaling Pathway + 1.1147 × DNMT3A + 0.7829 × WBC. The risk score grouped IR-AML patients into two subgroups: intermediate-low risk (ILR, score < 1, n= 48) and intermediate-high risk (IHR, score ≥ 1, n= 62) groups (Table1). Concordance index[OS: 0.703, 95% CI (0.643, 0.763); RFS: 0.681, 95%CI (0.620, 0.741)] demonstrated well discrimination power and calibration plots showed that the nomograms did well compared with an ideal model. The 3-year OS for ILR and IHR groups were 72.3% and 29.4% (P < 0.0001), and 3-year RFS were 63.9% and 19.4% (P < 0.0001), respectively (Figure 1A-B). The similar results were also observed in TCGA cohort (3-year OS 57.7% vs. 26.2%, P= 0.019; Figure 1C). The survival was equivalent for patients with ILR when treated with chemotherapy and allogeneic hematopoietic stem cell transplantation (alloHSCT, 3-year OS: 74.7% vs. 70.8%, P= 0.936, RFS: 76.4% vs. 55.0%, P= 0.225; Figure 2A-B). However, alloHSCT benefited patients with IHR. Patients who received alloHSCT had survival advantages over those who treated with chemotherapy only (3-year OS: 51.6% vs. 20.7%, P= 0.022; 3-year RFS: 38.1% vs. 7.8%, P= 0.008; Figure 2C-D). The prognosis of patients with IHR was as poor as those with adverse-risk AML (n=65 , 3-year OS: 29.5% vs. 33.3%, P= 0.794; 3-year RFS: 19.4% vs. 39.1, P= 0.294).
Conclusions
In this study, we developed and validated a novel scoring model that incorporated molecular and clinical profiles. According to our score model, IR-AML patients could be further stratified into two subgroups with distinct clinical outcomes. And the prognosis of patients with IHR was similar with patients with adverse-risk. Moreover, alloHSCT would override the poor outcome of patients with IHR.
In summary, our scoring model might help identify patients with IR-AML who are most likely to benefit from alloHSCT. The results are needed to be validated in other independent cohorts and prospective studies before implementation into clinics.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal