Although the ELN MRD Working Party (Schuurhuis, 2018) recommends that Acute Myeloid Leukemia (AML) patients (pts) with mutated NPM1 (NPM1+) should have molecular assessment of measurable/minimal residual disease (MRD), the most clinically significant timepoints (TPs), the thresholds, the best source of samples, bone marrow (BM) or peripheral blood (PB) and especially the management of molecular relapse, remain controversial. We evaluated the prognostic significance of NPM1 molecular monitoring, its impact on disease recurrence and the outcome of salvage treatment.
From Jul 2010 to dec 2018, 83 consecutive NPM1+ AML pts (M/F: 44/39; median age 59 y, 27-74) eligible for intensive chemotherapy (i-T) were treated, according to the NILG-AML00 protocol (ClinicalTrial.gov: NCT00400673): ICE (idarubicine-ARAC-etoposide) as induction, followed by IC consolidation and 3 further consolidation cycles of high-dose ARAC. Allogeneic stem cell transplant (HSCT) was considered at relapse. Quantitative RT-PCR was performed to detect NPM1 mutation (Gorello, 2006) on BM and PB samples at diagnosis (TP0) and at given TPs: at complete remission (CR) (TP1), post-IC (TP2), post-1st ARAC consolidation (TP3) and at the end of treatment (TP4). Serial MRD monitoring during follow-up was made on PB every 3 months (mo) for 5 years (y) or until relapse. Molecular relapse (mR) was defined as the NPM1 level increase at least 1 log in 2 consecutive samples, in absence of hematological relapse (hR).
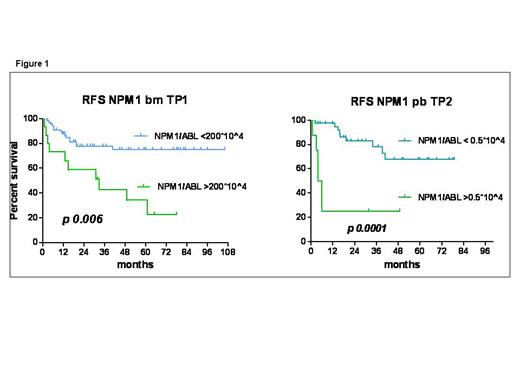
At baseline, karyotype (K) was abnormal in 8 pts (9.6%); 25 (30%) were FLT3-ITD mutated with a median Allelic Ratio (AR) of 0.35 (range: 0.2 to 2.18) (Pratcorona, 2013). During treatment, 608 samples were studied (383 BM and 225 PB), for a median of 8 per patient (range: 3 to 10). At TP1, a higher level of NPM1 unfavorably impacted on relapse (p 0.02) and a cut-off >200 *10^4/ABL was significantly associated with a higher relapse risk (RR) (68.7%) and lower RFS (p 0.006). Moreover, MRD positivity (value >0*10^4 NPM1/ABL) at TP2 on PB was associated to a higher RR (38.8%; p 0. 041) and a level >0.5*10^4/ABL allowed to predict relapse in 75% of pts, also impacting on RFS (p 0.0001) (Figure 1).Molecular NPM1 relapse/progression occurred in 10 pts early during treatment, after a median of 3.5 mo (1.4-6) from diagnosis and in 8 of them simultaneously with hR. In 16 pts mR occurred after a median CR duration of 18.5 mo (10.5-61.5) (late relapse). Hence a mR was more frequent in late than in early relapse (87.5% vs 20%; p 0.001). Median survival was 8.3 mo in early relapsing pts and was not reached in late relapsing pts (p. 0.0002). Age, NPM1 level at TP0, FLT3-ITD mutation, ITD-AR or abnormal K did not impact on type of relapse (mR or hR), but FLT3-ITD was more frequent in early than late relapse (60% vs 18.7%; p 0.04). After a median follow-up of 42 mo (4-108), 5-y relapse-free survival (RFS) and overall survival were 64.2% (+/-6.5% SE) and 71.1% (+/-6.1% SE), respectively. Overall, considering both mR and hR, 26 pts (31.3%) relapsed after a median of 13.5 mo after CR; 24/26 pts received a salvage treatment and 14 (53.8%) (median age 49y, 27-64) could proceed to HSCT after a median of 2.9 mo (5 too old, 7 early progression). Salvage treatment before HSCT was i-T in 7/14 and non-i-T in 7/14 pts (2 ATRA and 5 D-actinomycin, Falini 2015) for a median of 3 cycles (range 2-4). Disease status at HSCT was: mCR in 3 pts, CR with detectable MRD (median NPM1: 125*10^4/ABL, range 17.8-3849) in 9, refractory in 2 pts. Nine/14 HSCT pts (64.2%) are alive and in remission at a median of 29.6 mo (13-49) from HSCT, 2 pts died of sepsis in c-GVDH at 6.2 and 37.5 mo after HSCT, 1 died of VOD and 2 of disease progression. At 3 months after HSCT all MRD positive pts were NPM1 negative. mR occurred in 3 pts at 8, 12 and 15 mo after HSCT and was successfully treated with early pre-emptive donor lymphocyte infusions. With the limits of small numbers, age, type (mR or hR) or timing of relapse (early or late), disease status at HSCT, donor source and conditioning intensity didn't influence survival.
Our study shows that PCR monitoring during treatment can identify pts at higher RR according to the transcript levels in BM and PB samples. In NPM1+ AML, molecular monitoring in PB during follow-up is of crucial importance in detecting late molecular relapse allowing to use less toxic molecular-oriented treatments as a bridge to HSCT. Further studies on larger cohorts hopefully will help to confirm its usefulness to guide the treatment approach.
Rossi:Astellas: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Mundipharma: Honoraria; BMS: Honoraria; Sandoz: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy; Roche: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees.
D-actinomicin
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal