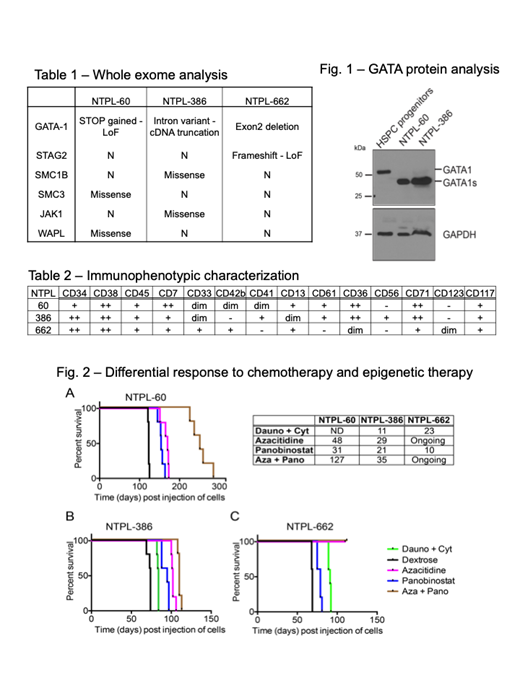
Children with Down syndrome (DS) are at a 500-fold increased risk for developing acute myeloid leukemia (AML) before they reach five years of age. DS-AML blasts have hypersensitivity to chemotherapeutic drugs such as cytarabine and daunorubicin. However, therapy-induced toxicity results in greater morbidity and remains a major barrier in attaining higher survival rate. Thus, alternate therapy approaches to minimize toxicity and increase efficacy are needed. Patient-derived xenograft (PDX) models of DS-AML are very limited and have not been used for preclinical studies. We generated three distinct disseminated PDX models by successful engraftment and serial passage of primary DS-AML blasts in NSG-B2m mice. DS-AML is characterized by the pathognomonic mutation in the gene encoding the essential hematopoietic transcription factor GATA1, resulting in N-terminally truncated mutant GATA1s protein. Accordingly, we identified GATA1 mutations and GATA1s protein (Fig. 1) in the DS-AML PDX lines. In addition to GATA1 mutation, whole exome sequencing also identified mutations in one or more co-operating mutations reported previously in DS-AML (Table 1). These PDX lines were extensively characterized and authenticated to be highly concordant with the primary patient sample with respect to immunophenotype (Table 2) and transcriptome analysis (by targeted sequencing using Archer FusionPlex HemeV2 panel), signifying the validity of the PDX models.
We evaluated the efficacy of cytotoxic chemotherapy and epigenetic therapy in mice transplanted with DS-AML PDX lines. Cytotoxic chemotherapy comprised daunorubicin 1.5 mg/Kg Qxd3 i.v. and cytarabine 50 mg/Kg Qxd5 i.p. Epigenetic therapy consisted of DNA hypomethylating agent azacitidine and HDAC inhibitor panobinostat, alone or in combination. NSG-B2m mice were injected with DS-AML PDX lines intravenously. Disease progression and engraftment of human cells was monitored by periodic determination of the percentage of human leukemic cells versus mouse cells in the peripheral blood by staining with species-specific fluorescent antibodies followed by flow cytometry. The mice were randomized into 5 treatment groups - 1) cytotoxic chemotherapy, 2) vehicle (5% dextrose), 3) azacitidine (2.5 mg/Kg), 4) panobinostat (2.5 mg/Kg), and 5) combination of azacitidine + panobinostat (2.5 mg/Kg each). The epigenetic drugs were dosed with four cycles lasting five days a week with two days rest. One-week rest period was included between cycles 2 and 3.
Chemotherapy prolonged survival by 11 and 23 days respectively in mice injected with NTPL-386 and NTPL-662. Single agent azacitidine and panobinostat prolonged survival by 48 and 31 days respectively in NTPL-60 mice. Mice transplanted with NTPL-386 showed enhanced survival by 29 and 21 days when treated with azacitidine and panobinostat alone. NTPL-662 study is ongoing and so far panobinostat treated mice showed 10 day increase in cell survival, while both azacitidine and azacitidine + panobinostat groups are alive and healthy. Azacitidine was more efficient than panobinostat in inducing remission and increasing the median survival compared to vehicle treated mice in all 3 models (Fig. 2A-C). Mice treated with the combination of azacitidine and panobinostat showed the greatest benefit and survived 127 days (for NTPL-60) and 55 days (for NTPL-386). Although azacitidine and panobinostat synergized to reduce leukemic burden and prolong survival in both PDX lines, there was a remarkable difference between the responses. Epigenetic therapy appeared to be more effective than chemotherapy. In conclusion, using DS-AML PDX models generated and characterized in-house, we show the efficacy of epigenetic therapy in comparison to chemotherapy.
Druley:Washington University: Employment; ArcherDX Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal