
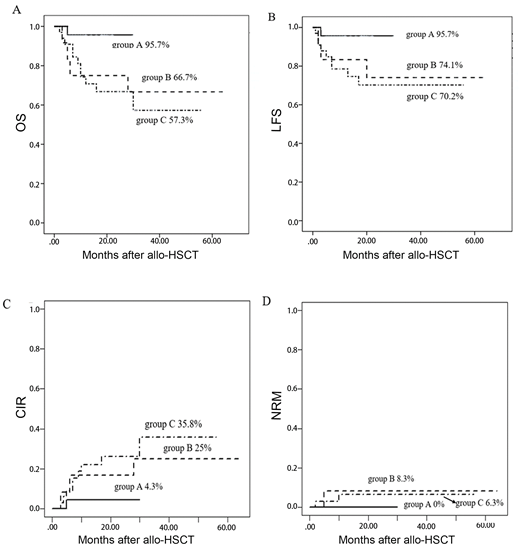
Background: Compared with traditional chemotherapy, allogeneic hematopoietic stem cell transplantation (allo-HSCT) can improve the prognosis of patients with FLT3-ITD positivity. Relapse after transplantation is still an important factor affecting survival. Prophylactic donor lymphocyte infusion (DLI) and maintenance sorafenib are two methods for preventing relapse after allo-HSCT in FLT3-ITD-positive acute myeloid leukemia (AML) patients, and their roles in these patients after allo-HSCT need to be further studied. Methods: From January 2014 to December 2018, 69 FLT3-ITD-positive patients who received allo-HSCT at our center were included in this retrospective study. All patients were divided into four groups according to the different treatments received: group A (23 patients who received maintenance sorafenib), group B (12 patients who received prophylactic DLI), group C (33 patients who received neither prophylactic DLI nor maintenance sorafenib), and group D (1 patient who received a combination of prophylactic DLI with maintenance sorafenib), because there was only one patient in group D, it was not included in the analysis. Results: For all patients, the 2-year estimated overall survival (OS) and leukemia-free survival (LFS) were 79.9% and 78.3%, respectively, and the 2-year cumulative incidence of relapse (CIR) was 17.2%. The 2-year estimated OS, LFS and CIR in group A, group B and group C were 95.7%vs75%vs66.8%, 95.7%vs74.1%vs70.2% and 4.3%vs6.7%vs26.1%, respectively. Group A was the best one in OS, LFS and CIR. Between the group A and group C, there were significant difference in OS(P=0.03) and LFS(P=0.04), but not in CIR(P=0.06).The OS in group A was significantly higher than group B(P=0.047), but the difference was not statistically in LFS and CIR (P=0.14 and P=0.21). Conclusion: Maintenance sorafenib after allo-HSCT was associated with improved outcomes for FLT3-ITD positive AML patients and was superior to prophylactic DLI in promoting FLT3-ITD-positive patients obtain a better survival after allo-HSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal