Background:
Patients with favorable risk acute myeloid leukemia (AML) by European LeukemiaNet (ELN) criteria are treated with intensive chemotherapy that yields high remission rates and is often curative. Intensive chemotherapy typically includes cytarabine and anthracycline (7+3) with high-dose daunorubicin (dauno 90mg/m2). When compared to 45mg/m2, dauno 90mg/m2 yields superior survival in younger, favorable risk AML patients. Since the re-approval of gemtuzumab ozogamicin (GO), standard practice now incorporates GO in the 7+3 backbone for favorable risk disease, however, it is typically with dauno 60mg/m2 rather than 90mg/m2. We aim to describe responses after 7+3 (dauno 90mg/m2) and 7+3 (dauno 60mg/m2) plus GO (7+3GO) in favorable risk AML.
Methods:
We retrospectively annotated 56 favorable risk AML patients (pts) who received upfront intensive chemotherapy at Moffitt Cancer Center between 2013 and 2019. They were divided in two cohorts: Cohort A) 7+3 with dauno 90mg/m2; Cohort B) 7+3 with dauno60mg/m2 plus GO at any time during induction or consolidation. Clinical and molecular data were abstracted for each patient in accordance with Institutional Board Review approved protocol. Overall response rates (ORR) included pts achieving complete remission (CR) with minimal residual disease negativity (CRMRD-), CR, CR with incomplete count recovery (CRi). MRD testing included quantitative Real-Time polymerase chain reaction (qRT-PCR) of t(8;21), inv(16) and nucleophosphin 1 (NPM1) tested at least after 2 cycles of intensive chemotherapy. Fisher's Exact method was used to determine significance for categorical variables. All p-values are two-sided.
Results:
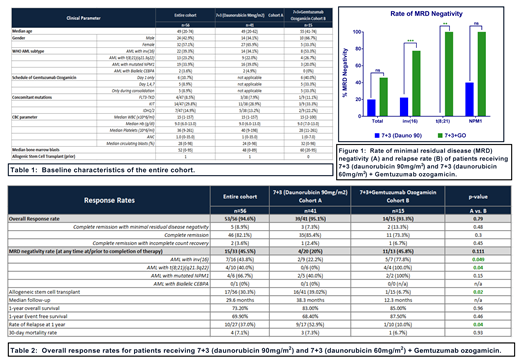
Fifty-six pts were analyzed, including 41 in cohort A and 15 in cohort B. Pt demographics are noted in Table 1. In cohort B, 40.0% received GO on day 1 only (3mg/m2, capped or uncapped), 33.3% received fractionated dosing (3mg/m2 days 1,4,7) and 33.3% received GO during consolidation only. 39.3% of pts had inv(16), 23.2% had t(8;21)(q21.3q22) and 33.9% had mutated NPM1.
In all 56 pts, ORR was 94.6%. ORR was 95.1% in cohort A and 93.3% in cohort B (p=0.79). CR rates were 85.4% in cohort A and 73.3% in cohort B (p=0.3) (Table 2). Rate of CRMRD- at any point was higher in cohort B vs cohort A, but did not reach statistical significance (45.8% vs. 20%, p=0.11). CRMRD- was significantly higher in pts with core binding factor (CBF) leukemia in cohort B vs cohort A (81.8% vs. 18.2%, p=0.008). The improvement in CRMRD- was seen in both inv(16) (A vs. B: 22.2% vs. 77.8%, p=0.049) and t(8;21) (0% vs. 100%, p=0.040). In pts with NPM1 mutations, a trend toward higher CRMRD- rates was noted in cohort B but this did not reach statistical significance (A vs. B: 40.0% vs. 100%, p=0.15).
Although the median follow up time in cohort B is significantly shorter (12.3 mos in cohort B vs 38.3 mos in cohort A), no difference was observed in the 1-year EFS (68.4% in cohort A and 87.5% in cohort B (p=0.46)) or OS (83.0% in cohort A and 89.0% in cohort B (p=0.96)). No difference was seen in early mortality (30-day) between the two arms (7.3% and 6.7% in cohorts A and B, respectively (p=0.93)).
Conclusions:
We demonstrate that incorporation of GO to 7+3 with dauno 60mg/m2 yields comparable remission rates to 7+3 with dauno 90mg/m2. Importantly, GO based regimens produce higher rates of MRD negativity compared to 7+3 with dauno 90mg/m2 in CBF leukemia. Longer follow up is needed in order to accurately assess the impact of GO based regimens on overall survival outcomes and whether GO-induced higher MRD negativity rates will translate into superior survival for patients with favorable-risk cohort when compared to 7+3 with high dose daunorubicin.
Talati:Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Pfizer: Honoraria; Celgene: Honoraria; Agios: Honoraria; Daiichi-Sankyo: Honoraria; Astellas: Honoraria, Speakers Bureau. Komrokji:celgene: Consultancy; pfizer: Consultancy; DSI: Consultancy; Incyte: Consultancy; Agios: Consultancy; JAZZ: Consultancy; Novartis: Speakers Bureau; JAZZ: Speakers Bureau. Kuykendall:Janssen: Consultancy; Abbvie: Honoraria; Incyte: Honoraria, Speakers Bureau; Celgene: Honoraria. Padron:Incyte: Research Funding. List:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Sallman:Celyad: Membership on an entity's Board of Directors or advisory committees. Sweet:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Stemline: Consultancy; Agios: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Jazz: Speakers Bureau; Celgene: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding. Lancet:Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Other: fees for non-CME/CE services ; Agios, Biopath, Biosight, Boehringer Inglheim, Celator, Celgene, Janssen, Jazz Pharmaceuticals, Karyopharm, Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal