
Introduction: Patients (pts) with relapsed/refractory (R/R) acute myeloid leukemia (AML) with FLT3-ITD mutations have a dismal prognosis and limited treatment options. Given the aggressiveness of FLT3-ITD AML, therapies that produce rapid and sustained disease control are needed. Quizartinib (Q), a once-daily, oral, highly potent and selective FLT3 inhibitor, demonstrated a clinically meaningful overall survival (OS) benefit in pts with R/R FLT3-ITD AML vs salvage chemotherapy (SC; 6.2 vs 4.7 mo [HR, 0.76 (95% CI, 0.58-0.98); P = .02]) in the phase 3 QuANTUM-R trial (NCT02039726; Cortes et al. Lancet Oncol, 2019). In this post hoc analysis, we characterize clinical outcomes in pts who achieved a composite complete remission (CRc) in QuANTUM-R, including pts with CR with incomplete hematologic recovery (CRi) and transfusion independence.
Methods: Pts aged ≥ 18 years with FLT3-ITD AML R/R after standard AML therapy, with or without hematopoietic stem cell transplant (HSCT), were randomized 2:1 to Q (60 mg [30-mg lead-in]) or 1 of 3 prespecified SC regimens. Pts receiving HSCT in the Q arm could resume Q after HSCT. Response was assessed per modified International Working Group criteria (Table 1). Transfusion dependence at baseline (BL) was defined as any platelet (PLT) or red blood cell (RBC) transfusion within 28 days of first dose. Post-BL transfusion independence was defined as no PLT or RBC transfusions for any consecutive 56-day period on treatment.
Results: Of 367 randomized pts, 245 and 122 were randomized to Q and SC, respectively. Median duration of treatment was 97 days with Q (including post-HSCT resumption) and ranged from 5 to 10 days with SC depending on the regimen. Median follow-up was 23.5 mo.
In the intent-to-treat population, CRc was consistent across prespecified subgroups (eg, sex, response to prior therapy, FLT3-ITD variant allele frequency). Median time to CRc was 1.1 mo with Q and 0.9 mo with SC; median duration of CRc was 2.8 mo with Q and 1.2 mo with SC (Table 2). Most responses in both arms were CRi, achieved in 99 pts (40%) in the Q arm and 32 pts (26%) in the SC arm. Peripheral blood counts in pts who achieved CRi are shown in Table 3. Of pts with CRi, 45 (Q) and 13 (SC) underwent HSCT. Outcomes with HSCT included engraftment failure in 11% (Q), rejection in 2% (Q), relapse in 33% (Q) and 46% (SC), and successful transplant in 47% (Q) and 46% (SC) and were unknown in 7% (Q) and 8% (SC).
Of the 205 pts in the Q arm who were transfusion dependent at BL, 46 (22%) became transfusion independent post-BL (29 with HSCT; 17 without). Mean (SD) duration of post-BL transfusion independence was 255 (216.6) days. By response, 24 of 91 pts (26%) with CRi, 7 of 48 (15%) with a partial response (PR), and 4 of 47 (9%) with no response (NR) became transfusion independent post-BL; respective mean (SD) durations were 297.6 (268.3), 84.6 (21.7), and 211.3 (111.9) days. Of the 36 pts in the Q arm who were transfusion independent at BL, 20 (55.6%) maintained transfusion independence post-BL (12 with HSCT; 8 without; mean [SD] duration, 208 [203.1] days). Transfusion independence could not be assessed in the SC arm. Transfusion data was not collected after the end of treatment, and most pts in the SC arm were treated for < 56 days.
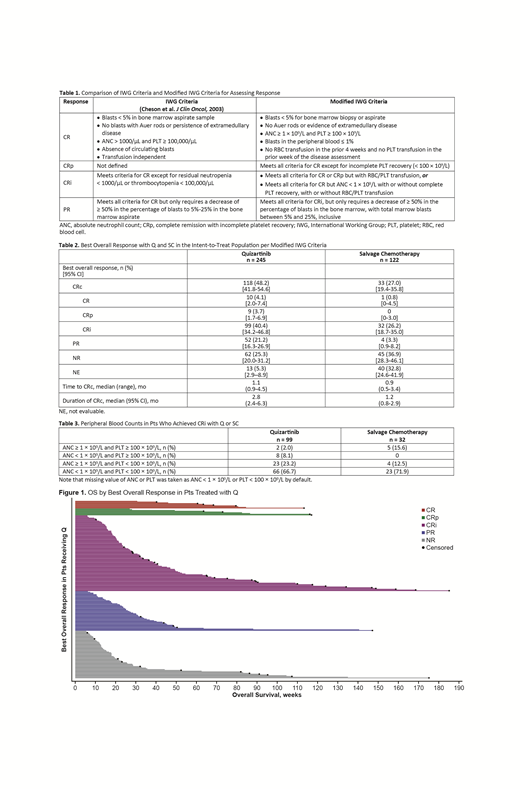
In the Q arm, median OS (95% CI) was longer in pts with CRi (7.5 [5.4-9.9] mo) vs pts with PR (6.1 [5.1-7.2] mo) or NR (4.1 [3.3-5.9] mo) (Figure 1); respective medians in the SC arm were 8.8 (6.3-20.8), 7.8 (5.4-28.2), and 3.8 (2.7-4.7) mo. In the 34 pts in the Q arm with a last response of CRi prior to allogeneic HSCT, median OS (95% CI) was 25.1 (9.9-NA) mo; in the SC arm (n = 9), median OS (95% CI) was 20.1 (4.3-NA) mo. In the Q arm, median OS (95% CI) was longer in pts with CRi who became transfusion independent post-BL vs pts who did not (27.1 [10.4-NA] vs 5.3 [4.6-6.4] mo); similarly, median OS (95% CI) was longer in pts who maintained transfusion independence vs pts who did not (25.1 [11.4-NA] vs 9.6 [4.6-NA] mo).
Conclusions: Q induced rapid, durable, and consistent tumor control in pts with FLT3-ITD R/R AML irrespective of prior therapy, including HSCT. A high proportion of pts with CRi became eligible for HSCT, the only potential curative treatment option. Moreover, a high proportion achieved durable transfusion independence regardless of HSCT, which is also associated with clinical benefit. These data highlight the clinical benefit of achieving CRi with Q in pts with FLT3-ITD R/R AML, a population with a high unmet need.
Levis:Novartis: Consultancy, Research Funding; Daiichi Sankyo Inc: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Astellas: Consultancy, Research Funding; Amgen: Consultancy, Honoraria; FUJIFILM: Consultancy, Research Funding; Menarini: Consultancy, Honoraria. Ganguly:Daiichi Sankyo: Research Funding; Seattle Genetics: Speakers Bureau; Kite Pharma: Honoraria, Other: Advisory Board; Janssen: Honoraria, Other: Advisory Board. Khaled:Alexion: Consultancy, Speakers Bureau; Daiichi Sankyo: Other: Travel support; Omeros: Consultancy. Krämer:BMS: Research Funding; Bayer: Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees. Martinelli:Novartis: Consultancy, Other: trial grant; Pfizer: Consultancy, Other: trial grant; Roche: Consultancy, Other: trial grant; Incyte: Consultancy, Other: trial grant; Ariad: Consultancy, Other: trial grant; Daiichi Sankyo: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Other: trial grant; Amgen: Consultancy, Other: trial grant; Abbvie: Consultancy, Honoraria, Other: trial grant; Janssen: Consultancy, Other: trial grant. Perl:BioMed Valley Discoveries: Research Funding; FujiFilm: Research Funding; Novartis: Honoraria, Other: Advisory board, Non-financial support included travel costs for advisory board meetings as well as a medical writing company that assisted with manuscript preparation/submission and slide deck assembly for academic meeting presentations of the data., Research Funding; Takeda: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; Bayer: Research Funding; NewLink Genetics: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; Actinium Pharmaceuticals: Consultancy, Honoraria, Other: Clinical Advisory Board member, Research Funding; Agios: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Non-financial support included travel costs for advisory board meetings.; Jazz: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; Astellas: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings as well as a medical writing company that assisted with manuscript preparation/submission and slide deck assembly for academic meeting presentations of trial data., Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Other, Research Funding; Arog: Consultancy, Other: Non-financial support included travel costs for advisory board meetings.; AbbVie: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.. Russell:Astellas: Consultancy, Honoraria, Speakers Bureau; DSI: Consultancy, Honoraria, Speakers Bureau; Pfizer Inc: Consultancy, Honoraria, Speakers Bureau; Jazz: Consultancy, Honoraria, Speakers Bureau. Choi:Daiichi Sankyo: Employment. Lesegretain:Daiichi-Sankyo Inc.: Employment, Equity Ownership. Mendell:Daiichi Sankyo, Inc.: Employment. Mires:Daiichi Sankyo: Employment. Zhang:Daiichi Sankyo: Employment. Cortes:Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; BiolineRx: Consultancy; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Biopath Holdings: Consultancy, Honoraria; Takeda: Consultancy, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal