Background: Acalabrutinib (ACP-196) is a highly selective, potent Bruton tyrosine kinase (BTK) inhibitor developed to minimize off-target activity. Acalabrutinib monotherapy shows promising safety and efficacy in CLL (Ghia et al 2019). However, a few patients (pts) develop resistance to acalabrutinib. A known mechanism of covalent BTK inhibitor resistance is acquired mutations in BTK (particularly Cys481) and its downstream target PLCg2. Alternate mechanisms and the contribution of the CLL microenvironment to acquired resistance remain to be elucidated. In this study, we performed cell surface phenotyping, intracellular signaling, and RNA-seq analyses on samples from 39 pts with relapsed/refractory or treatment-naive CLL from the ACE-CL-001 clinical trial (NCT02029443) to identify novel mechanisms of acalabrutinib resistance with focus on the CLL microenvironment.
Methods: Pts were divided into 2 groups: those who continued to respond to treatment (non-progressed, NP, n=23) and those who developed progressive disease (progressed, PD, n=16) within 36 months of starting acalabrutinib. Blood mononuclear cells (PBMCs) were analyzed after 6 months of acalabrutinib therapy (100 mg twice a day) for the first (NP) group of patients, or at the time of progression for the second (PD) group of patients and compared with pre-treatment baseline. Expression of cell surface markers, including CD49d, CD38, and CD79b, was evaluated by flow cytometry. A 30% positive cut-off was used to identify pts that express high levels of CD49d, an α-chain of the VLA-4 integrin (Tissino, et al 2018), CD38, and CD79b. Intracellular flow cytometry was used to measure cell proliferation via Ki-67 staining. RNA-seq was used to measure changes in gene expression.
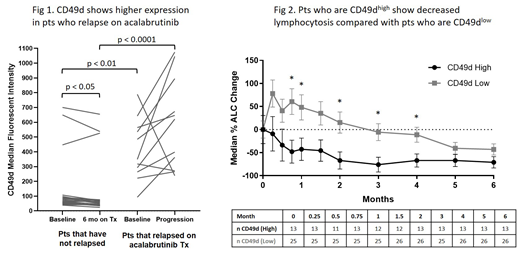
Results: Cell surface phenotyping showed higher expression of CD49d (p<0.001) and CD79b (p<0.01) at baseline and after treatment (p<0.0001; p=0.017) in pts who progressed compared with pts who continued responding to treatment (Fig 1). CD49d levels decreased in NP pts 6 months after treatment (p=0.016) but remained high in pts who progressed. Increased surface expression of CD49d was associated with increased surface expression of CD38 (p<0.001). There was strong correlation between cell surface expression of CD49d and its transcript ITGA4 by RNA-seq (R=0.78, p<0.0001). Gene expression of CD38 and CD79B also correlated with their protein expression (R=0.8, p<0.0001 and R=0.42, p<0.001). CD49d high pts showed decreased lymphocytosis with lower % change in median absolute lymphocyte count from baseline to 5 months post-treatment, compared with CD49d low pts (Fig 2). CD49d high pts also showed a trend of inferior nodal response with less tumor reduction compared with CD49d low pts. Additionally, high CD49d expression was associated with increased risk of progressive disease in relapsed/refractory pts compared with low CD49d expression (HR=3.66, p=0.045). Previous studies with another BTK inhibitor, ibrutinib, also identified a correlation between expression of CD49d and reduced lymphocytosis, nodal response, and progression-free survival in pts with CLL (Tissino, et al 2018). High CD49d cell surface expression was correlated with increased Ki-67 at baseline and after acalabrutinib treatment (p=0.012, p<0.001). Ki-67 levels decreased in CD49d low pts (p<0.0001), but not in CD49d high pts, after acalabrutinib treatment. CD49d may contribute to acalabrutinib resistance in CLL by enhancing cell survival through adhesion to the tumor microenvironment and may serve as a predictive marker to identify pts at high risk of developing resistance to acalabrutinib.
Conclusions: High surface expression of CD49d (VLA-4) and CD79b prior to and after therapy correlates with acalabrutinib resistance in pts with CLL. Targeting CD49d may prove an effective strategy to overcome acalabrutinib resistance in CD49d high pts.
Bibikova:Acerta Pharma: Employment, Equity Ownership; AstraZeneca: Equity Ownership. Law:Acerta Pharma: Employment; AstraZeneca: Equity Ownership. Clevenger:Acerta Pharma: Employment; AstraZeneca: Equity Ownership. Cheung:AstraZeneca: Equity Ownership; Acerta Pharma: Employment, Equity Ownership. De Jesus:Acerta Pharma: Employment, Equity Ownership; AstraZeneca: Equity Ownership. Gulrajani:Acerta Pharma: Employment, Equity Ownership; AstraZeneca: Equity Ownership. Yamaguchi:AstraZeneca: Equity Ownership; Acerta Pharma: Employment. Do:Acerta Pharma: Employment, Equity Ownership; AstraZeneca: Equity Ownership. Burke:AstraZeneca: Employment, Equity Ownership. Brock:AstraZeneca: Equity Ownership; Acerta Pharma: Employment. Munugalavadla:Gilead Sciences: Equity Ownership; AstraZeneca: Equity Ownership; Acerta Pharma: Employment. Frigault:Acerta Pharma: Employment; AstraZeneca: Employment, Equity Ownership. Byrd:Acerta: Research Funding; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau; Genentech: Research Funding; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau; Novartis: Other: Travel Expenses, Speakers Bureau; Genentech: Research Funding; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau; Janssen: Consultancy, Other: Travel Expenses, Research Funding, Speakers Bureau; BeiGene: Research Funding; BeiGene: Research Funding; Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau; BeiGene: Research Funding; Gilead: Other: Travel Expenses, Research Funding, Speakers Bureau; Novartis: Other: Travel Expenses, Speakers Bureau; Genentech: Research Funding; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau; Acerta: Research Funding; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; TG Therapeutics: Other: Travel Expenses, Research Funding, Speakers Bureau; Acerta: Research Funding; Ohio State University: Patents & Royalties: OSU-2S; Pharmacyclics LLC, an AbbVie Company: Other: Travel Expenses, Research Funding, Speakers Bureau; Ohio State University: Patents & Royalties: OSU-2S; Novartis: Other: Travel Expenses, Speakers Bureau; Ohio State University: Patents & Royalties: OSU-2S. Furman:Acerta Pharma: Consultancy; AstraZeneca: Consultancy; Incyte: Consultancy; Janssen: Consultancy; Beigene: Consultancy; Oncotracker: Consultancy; Pharmacyclics: Consultancy; Sunesis: Consultancy; TG Therapeutics: Consultancy; Verastem: Consultancy; Genentech: Consultancy; Abbvie: Consultancy. Brown:Catapult Therapeutics: Consultancy; AbbVie: Consultancy; Genentech/Roche: Consultancy; Acerta Pharma: Consultancy; Pharmacyclics: Consultancy; Pfizer: Consultancy; Janssen: Honoraria; Teva: Honoraria; AstraZeneca: Consultancy; Octapharma: Consultancy; Invectys: Other: Data safety monitoring board; Morphosys: Other: Data safety monitoring board; Dynamo Therapeutics: Consultancy; BeiGene: Consultancy; TG Therapeutics: Consultancy; Sunesis: Consultancy; Loxo: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Juno/Celgene: Consultancy; Gilead: Consultancy, Research Funding; Verastem: Consultancy, Research Funding; Sun Pharmaceuticals: Research Funding; Novartis: Consultancy. Covey:AstraZeneca: Equity Ownership; Acerta Pharma: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal