Abstract:
Polo-like kinase 4 (PLK4) is a serine/threonine kinase that plays a critical role in regulating centriole duplication. PLK4 overexpression causes centriole amplification and genomic instability, which is the hallmark of cancer. Thus, PLK4 has been suggested as a tumor promoter role in solid malignancies. CFI-400945 is a highly selective small-molecule inhibitor of PLK4. CFI-400945 treatment results in centriole duplication defects, which leads to mitotic catastrophe and cell death. The antineoplastic effects of CFI-400945 have been reported in many cancers and this inhibitor has entered Phase I clinical trials for solid tumors. However, the role of PLK4 and cellular effects of CFI-40045 in hematological malignancies remains elusive.
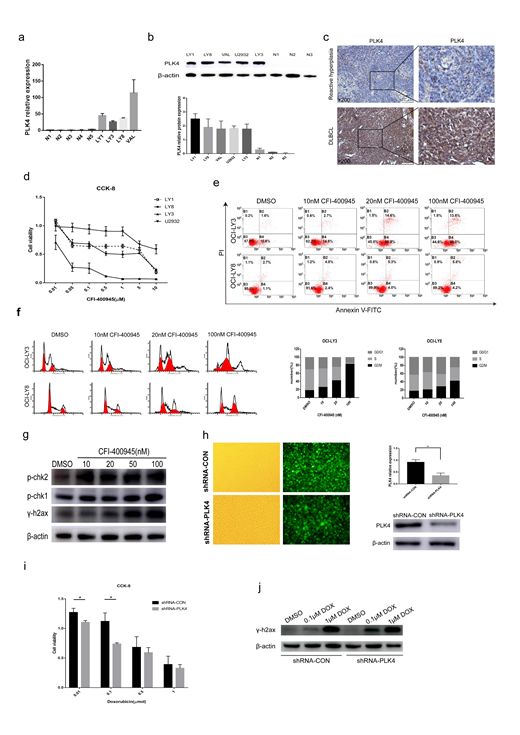
In the present study, we found that PLK4 expression is up-regulated at both transcriptional and translational levels in diffuse large B-cell lymphoma (DLBCL) cell lines (Fig. 1a, Fig. 1b). DLBCL patients displayed high protein levels of PLK4 as visualized by immunohistochemistry (Fig. 1c). In vitro, treatment of DLBCL cell lines with CFI-400945 induced a decrease in cell proliferation and an increase in cell apoptosis and a block at the G2-M transition in a dose-dependent manner (Fig. 1d, Fig. 1e, Fig. 1f). Furthermore, we found that the antineoplastic effects of CFI-400945 in DLBCL cell lines were due to the increased DNA damage, as observed by increased accumulation of γH2AX through phosphorylation of CHK1/CHK2 (Fig. 1g). Since PLK4 inhibition affects DNA damage, we next aimed to explore the effects of PLK4 expression on sensitivity of DNA damage-inducing drugs (e.g., doxorubicin). We found that silencing of PLK4 via shRNA improve the anti-tumor effects of doxorubicin in DLBCL cell lines (Fig. 1h, Fig. 1i). PLK4 inhibition in combination with doxorubicin significantly increased phosphorylation of H2AX compared to cells treated with doxorubicin alone (Fig. 1j).
In summary, our data indicates that PLK4 is aberrantly expressed in DLBCL cell lines and tissues. Targeting PLK4 with the selective inhibitor CFI-400945 suppresses cell proliferation and induces apoptotic deaths and causes DNA damage in vitro. Our findings established PLK4 as a potential therapeutic target in DLBCL. Silencing of PLK4 expression induces accumulation of DNA damage and enhances the cytotoxic effects of doxorubicin. Thus, the levels of PLK4 may serve as a determinant of doxorubicin sensitivity and a predictive biomarker of the patients with DLBCL treated with R-CHOP based therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal