Introduction
Colony stimulating factor 3 receptor (CSF3R) plays an important role in granulocyte proliferation and differentiation. Acquired mutations in CSF3R are found in the majority of cases of chronic neutrophilic leukemia and in a minority of atypical chronic myeloid leukemia and acute myeloid leukemia patients, whereas inherited biallelic mutations have been identified as a cause of severe congenital neutropenia (SCN). CSF3R variants have also been detected in lymphoid cells in patients with SCN. However, whether germline monoallelic CSF3R variants predispose to myeloid and/or lymphoid malignancies is unknown.
Methods
We analyzed variants detected by a clinical next generation sequencing (NGS) panel of about 150 genes important in oncogenesis (OncoPlus) performed on peripheral blood and/or bone marrow of patients with hematopoietic malignancies at The University of Chicago Medical Center from June 2017 to February 2019. We prioritized genes for which variants were likely of germline origin based on variant allele fraction (VAF) > 0.4 and persistence over multiple tests. Variant germline status was determined by extracting DNA from cultured bone marrow-derived mesenchymal stromal cells and/or from cultured skin fibroblasts. Variants in CSF3R [transcript NM_000760.3] were ranked by predicted pathogenicity using standard American College of Medical Genetics criteria. Variants of uncertain significance (VUS) were evaluated using population frequency data, in silico functional prediction, and REVEL scores.
Results
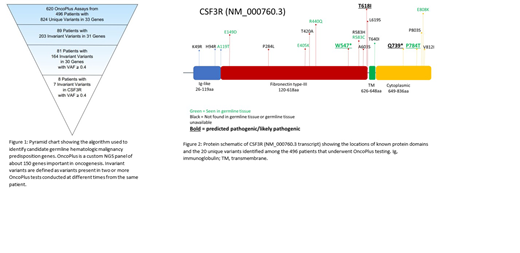
A total of 620 OncoPlus tests were conducted on 496 patients (ages 1 month - 96 years; median 64 years), and 824 unique variants were identified within 33 genes. Among these patients, 89 (17.9%) had an invariant variant, one that was found at similar VAFs across multiple testing time points. These invariant variants involved 31 different genes with a total of 203 unique variants. Variants with a VAF less than 0.4 were excluded from further study, leaving 81 patients with 164 invariant variants among 30 genes (Figure 1). The genes with the most invariant variants included known germline predisposition genes, such as ATM, BRCA2, DDX41, ETV6, and TP53, as well as CSF3R, which was not previously recognized as an autosomal dominant germline cancer susceptibility gene. Among the 81 patients, 8 (9.9%) were found to have invariant variants in CSF3R, with 7 unique variants identified. Including patients for whom only a single OncoPlus test was conducted in addition to those with invariant variants, there were a total of 43 patients with 20 unique variants in CSF3R among the entire 496 patient cohort (Figure 2).
Of these 20 CSF3R variants, 8 (40%) were found to be germline, and among those, one was classified as likely pathogenic, one was classified as a VUS, and six were classified as likely benign. We focused our attention on the likely pathogenic and VUS germline variants. These two variants were among those with the highest predicted pathogenicity scores. The likely pathogenic variant, W547*, is a nonsense mutation in the extracellular domain of CSF3R that was identified in a patient with a history of bladder cancer treated with surgery and chemotherapy who subsequently developed therapy-related myelodysplastic syndrome. W547* has previously been found to be pathogenic in a compound heterozygous state in an infant with SCN. The germline VUS, P784T, is a missense variant identified in a patient with multiple myeloma. This variant localizes to the cytoplasmic domain of CSF3R and is not listed in the COSMIC or gnomAD databases. Functional studies are underway to assess the oncogenic potential of the W547* and P784T variants.
Conclusion
Detection of invariant variants on clinical, tumor-based NGS panels can be used as a way to identify potential germline mutations to aid in new germline syndrome discovery. Using this approach, CSF3R was identified as a candidate gene for which monoallelic pathogenic variants may predispose to both myeloid and lymphoid malignancies. Future work is ongoing to assess the functional consequences of germline CSF3R variants and the frequency of such mutations.
Avalos:Best Practice-Br Med J: Patents & Royalties: receives royalties from a coauthored article on evaluation of neutropenia; Juno: Membership on an entity's Board of Directors or advisory committees. Godley:Invitae, Inc.: Membership on an entity's Board of Directors or advisory committees; UpToDate, Inc.: Patents & Royalties: receives royalties from a coauthored article on inherited hematopoietic malignancies .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal