
Introduction: Hematopoiesis is severely reduced in severe aplastic anemia (SAA) probably due to immunological destruction of hematopoietic stem and progenitor cells. Bone marrow transplantation is preferred as first-line therapy in patients younger than 40 years with a matched related sibling donor. For those who are not candidate for transplant, immunosuppressive therapy with horse antithymocyte globulin (hATG) plus cyclosporine (CsA) remains the standard of care. However, hATG was discontinued in most Asian, South American, and European countries with only rabbit ATG (rATG) available. rATG is a more potent immunosuppressant which has associated with a worst hematological response and survival at 6 months in prospective studies. Using rATG in first-line therapy appears to add little to what has been observed with CSA alone. Alemtuzumab (ALZ), a humanized anti-CD52 monoclonal antibody is also active in AA due to its lymphocytotoxic properties. The use of ALZ in monotherapy has shown an overall response rate (ORR) comparable to that of r-ATG (37% and 33%, respectively) when applied as salvage (following hATG failure) in a randomized study. Other experiences which combined ALZ and CsA led to a 58% ORR in SAA patients after a cumulative dose of 103 mg of subcutaneous (SC) ALZ. Here we describe a multicenter retrospective analysis of SC ALZ in association with CsA for patients with AA.
Methods: We retrospectively analyzed all patients who received outpatient SC ALZ for the treatment of aplastic anemia in 2 centers: Universidade Federal de Sao Paulo (Sao Paulo-BRAZIL) and Leeds Teaching Hospitals, UK from March 2009 until March 2019. In Sao Paulo, alemtuzumab total dose was 103 mg in an escalating dose of 3-10-30-30-30 mg, except for three elderly patients who received a total dose of 73. In Leeds-UK, total dose varied from 120 mg to 150 mg. The primary outcome was overall response rate (ORR) at six months. Median follow up was 31 months (range 4-110 months).
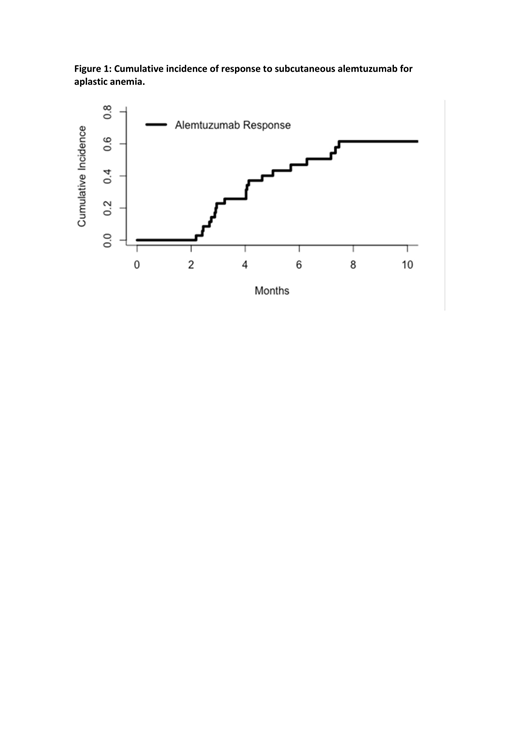
Results: We identified 35 treatments with SC ALZ in 32 AA patients of which 78% had SAA or very severe aplastic anemia. Calcineurin inhibitors were used in association with ALZ in 80% of cases (cyclosporine in 26 cases, tacrolimus in 2 cases). Median age was 44 years (range: 18-79), and nineteen patients (59%) were male. Seventeen patients (53%) were treatment-naïve, and six patients (19%) had one prior line of therapy. Nine patients (28%) were relapsed/refractory, and received ALZ after at least two lines of prior therapy. Seventeen patients (53%) presented a PNH clone at diagnosis. Median time between diagnosis and ALZ treatment was 6 months (range 1-293). No treatment-related serious adverse events were observed. Infectious complications were infrequent: there was only one case of successfully treated CMV reactivation and one case of fatal EBV-related infection. ORR at 6 months was 57% (complete response: 11%, partial response: 46%), with corresponding cumulative incidence of response of 47% at 6 months and 62% at 1 year (figure 1). ORR was 69% in treatment-naïve patients younger than 60 years. Overall survival was 72% at 2 years. Adverse events were of low grade and infectious events were infrequent.
Conclusion: Subcutaneous alemtuzumab appears to be a feasible, effective and safe alternative to hATG in patients with AA requiring immunosuppressive treatment, especially in centers with limited access to hATG.
Hill:Apellis: Honoraria; Roche: Honoraria; Akari: Honoraria; Alexion: Honoraria; Bioverativ: Honoraria; Novartis: Honoraria; Regeneron: Honoraria; Ra Pharma: Honoraria. Munir:AbbVie: Honoraria; Alexion: Honoraria; Gilead: Honoraria; Janssen: Honoraria; Novartis: Honoraria; Roche: Honoraria; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sunesis: Consultancy; Pharmacyclics: Other: TBC; Acerta: Membership on an entity's Board of Directors or advisory committees. Hillmen:Roche: Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Apellis: Research Funding; Gilead: Research Funding. Risitano:Achillion: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees; Samsung: Membership on an entity's Board of Directors or advisory committees; Biocryst: Membership on an entity's Board of Directors or advisory committees; Samsung: Membership on an entity's Board of Directors or advisory committees; Amyndas: Consultancy; Ra Pharma: Research Funding; Alnylam: Research Funding; Achillion: Research Funding; Alexion: Honoraria, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Alnylam: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biocryst: Membership on an entity's Board of Directors or advisory committees; Apellis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amyndas: Consultancy; Ra Pharma: Research Funding; Apellis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Research Funding, Speakers Bureau. Scheinberg:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer,: Speakers Bureau; Alexion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Celgene: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal