Glucocorticoids, such as dexamethasone and prednisone, are mainstays in the treatment of lymphoid malignancies and autoimmune diseases. To understand the mechanism of how glucocorticoids induce cell death, we integrated a comprehensive, genome-wide shRNA screen with differential gene expression analysis in B-cell acute lymphoblastic leukemia (B-ALL). In addition to activating pro-apoptosis genes and repressing anti-apoptosis genes, we found that repression of B-cell development genes contributes to dexamethasone-induced cell death in B-ALL. Similarly, elevated glucocorticoid levels brought on by stress in stroke victims causes lymphocytopenia by inducing of apoptosis in immature B-cells, but not hemopoietic stem cells or mature B-cells. Additionally, chronic stress has been associated with immune suppression and increased risk for autoimmune disease. Together these findings suggest that glucocorticoids play a normal role in B-cell development, and that elevated glucocorticoid levels in response to stress can alter that development.
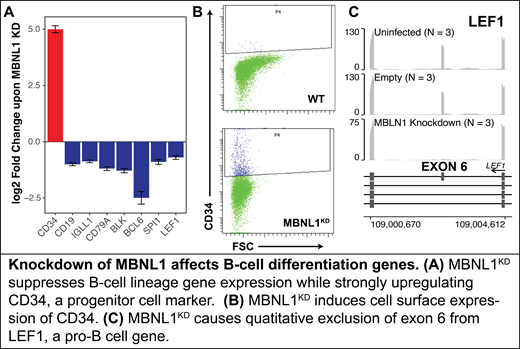
A control point in this mechanism may be MBNL1. MBNL1 is a sequence-specific RNA binding factor that alters mRNA splicing, stability, and localization. In this work, we show that glucocorticoid suppression of MBNL1 contributes to induction of cell death in B-ALL. Deletion of MBNL1 by CRISPR/Cas9 in the B-ALL cell line NALM-6 induces accumulation and depletion of mRNA levels for hundreds of genes. A large number of B-cell specification genes, including CD19, CD79A, SPI1, and LEF1, are repressed upon MBNL1 deletion, whereas the most highly activated gene is CD34, which is a marker for the less mature lymphoid progenitor cells. Depletion of MBNL1 also changed the splicing of B-cell development genes, including quantitative depletion of LEF1 exon 6. These data suggest that depletion of MBNL1 induces de-differentiation of B-cell precursors. Consistent with this model, an analysis of gene expression in differentiating B-cells revealed a rapid elevation of MBNL1 expression during B-cell specification (after the lymphoid precursor stage), and a concomitant repression of CELF2, an RNA-binding splicing factor that has been shown to oppose MBNL1. We therefore propose that glucocorticoids play a physiological role in B-cell development by restraining B-cell specification through repression of key genes, including MBNL1.
Tasian:Aleta Biotherapeutics: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Research Funding; Incyte Corportation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal