Introduction: Prevention of haemophilic arthropathy and quality of life´s (QoL) improvement are still the main goals in the haemophilia community. Haemophilic arthropathy is the result of clinical and subclinical bleeding during everyday activities and/or traumatic situations. Prophylaxis with extended half-life (EHL) factor replacement therapy is understood as an improvement solution for factor VIII (FVIII) PK properties, as half-life (T1/2) and area under the curve (AUC), however few real world data are yet available. EHL improved pharmacokinetic (PK) properties might directly drive into a reduction of the bleeding risk during physical activity (both therapeutical or leisure) for a longer period of time, allowing an increase in QoL.
World Health Organization (WHO) has set recommendations focused on the benefits of moderate and intense physical activity to improve joint health as well as to prevent common pathologies (obesity, diabetes, hypertension, cancer, depression, anxiety) and even the risk of death (since the absence of physical activity is the 4th factor risk worldwide).
The aim of this study was to define a safe program of physical activity, sport and physiotherapy along the time according the PK profile of patients treated with the EHL rurioctocog alfa pegol.
Materials and methods: PK parameters (infusion frequency, dosage, T½, peak level, trough level at 24, 48 and 72 hours (NV24/NV48 and NV72), and time to reach 5%, 2% and 1% FVIII levels (T5,T2 and T1) were analyzed in patients with hemophilia A after switching from an standard half-life (SHL) FVIII to the EHL (rurioctocg alfa pegol) FVIII replacement therapy. Tailored physical activity and physiotherapy programmes in place during SHL treatment were re-evaluated after switching to the EHL according the new individual PK profile. The Functional Independence Score in Hemophilia (FISH) form was used to measure ambulation.
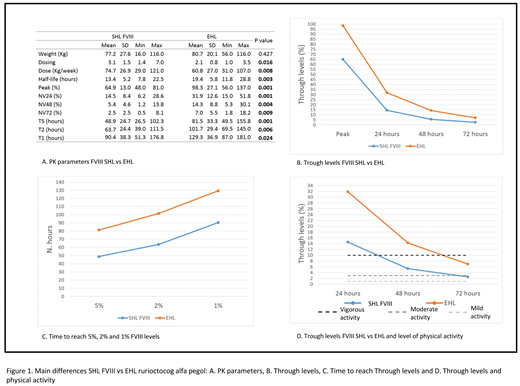
Results: Ten patients with hemophilia A (9 severe and 1 moderate) with a mean age of 34.49 (9.46) were analyzed. All the PK parameters evaluated showed a significant statistical improvement after the switch from an SHL to this FVIII EHL (Figure 1, A.). Specifically, higher T½, peak levels and trough levels were achieved using a lower dosage and infusion frequency (Figure 1, B).
After switching to the EHL, FVIII trough levels were higher than 5% until 3.4 days (81.5 hours) (Figure 1, C) post-infusion. This allowed us to establish a physiotherapy program as well as an intense-moderate physical activity program in patients without clinical evidence of bleeding events (Figure 1, D). The potentiation physical program to develop muscle tone for elbows, knees and ankles was authorized until the third day of EHL FVIII post-infusion. However, during SHL treatment, vigorous physical activity was never performed after 24 hours of FVIII post-infusion.
No joint bleeding events appeared during everyday physical activities nor during physiotherapy programs aimed to maintain joint trajectory and muscle power.
Regarding ambulation (FISH), all patients had hemophilic arthropathy in one joint. The results in the item "Run" were: 3 points for 3 patients (activity non comparable to normal activity), 2 points for 3 patients (requires an orthesis) and 1 point for 4 patients (activity not feasible).
Conclusions: EHL FVIII replacement with rurioctocog alfa pegol improved significantly all PK parameters compared to SHL factors. The administration of this EHL allows moderate and vigorous physical activity after more than 72 hours post-infusion, due to higher FVIII coverage along the time.
Querol:Baxalta US INC.: Research Funding. Pérez-Alenda:Baxalta US INC.: Research Funding. Megias:Baxalta US INC.: Research Funding; Grifols: Research Funding. Carrasco:Baxalta US INC.: Research Funding. Cid:Novo Nordisk: Honoraria; Shire, a Takeda company: Honoraria. Bonanad Boix:Baxalta US INC.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal