MDJ and MTJ contributed equally; FLL and AG contributed equally.
Introduction
Axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) are autologous anti-CD19 CAR T-cell therapies approved for the treatment of adults with relapsed or refractory large B-cell lymphoma (LBCL) who have failed at least two lines of systemic therapy. In the ZUMA-1 trial that led to axi-cel approval, the median time between apheresis and delivery of CAR T cells to the treating facility was 17 days (Neelapu, Locke et al. NEJM 2018). Bridging therapy, defined as lymphoma therapy given between apheresis and the start of lymphodepleting chemotherapy, was not permitted on ZUMA-1. By contrast, the pivotal JULIET trial for tisa-cel (Schuster et al. NEJM 2019) had a median time from enrollment to infusion of 54 days and 92% of patients received bridging therapy. Whether bridging therapy affects lymphoma CAR T outcomes is unknown. Here we evaluate patients receiving bridging therapy for axi-cel in a large multicenter cohort.
Methods and Results
The US Lymphoma CAR T Consortium includes seventeen US academic centers that contribute data from lymphoma patients treated with standard of care (SOC) CAR T-cell therapy independently of manufacturers. As of 8/31/2018, 300 patients were apheresed with intent to manufacture SOC axi-cel for LBCL. Of the 23 patients that underwent apheresis for axi-cel and did not receive it, 20 had lymphoma progression or death that precluded CAR T infusion, of which 16 received bridging therapy. In this study, we analyze the modified intent-to-treat (mITT) population of 276 patients receiving CAR T infusion with a median follow up of 9 months. In this group, 146 (53%) patients received bridging therapy while 130 (47%) patients received no bridging therapy. Bridging therapy consisted of steroids alone (n = 35, 24%), chemotherapy (n = 73, 50%), radiation (n = 24, 16%), or targeted therapies (n = 14, 10%).
At baseline, a higher proportion of patients in the bridging therapy group had an ECOG score of 2 - 4 vs. 0 - 1 (bridging 24.8%, no bridging 6.1%, p <0.001), IPI score of 3 -5 vs. 0 - 2 (bridging 67.6%, no bridging 34.3%, p <0.001), bulky disease greater than 10 cm (28.2% vs. 13.0%, p = 0.002), immunohistochemical double expression of MYC and BCL2 (42.5% vs. 24.6%, p =0.004) and would not have met all the eligibility criteria for ZUMA-1 for reasons other than bridging therapy (48.6% vs. 30.0%, p = 0.002).
After axi-cel infusion, patients in the bridging group had similar rates of severe (grade 3 or higher) cytokine release syndrome (CRS) (8.2% vs. 5.3%, p = 0.34) and ICANS (35.2% vs. 28.2%, p = 0.25). However, the rate of ICU admission was higher in the bridging group (41.4% vs. 22.9%, p = 0.001) as was median length of hospital stay (15 vs. 14 days, p = 0.02). While data on cytopenias was not collected, use of G-CSF after CAR T therapy was higher in the bridging group (48.2% vs. 32.1%, p = 0.006).
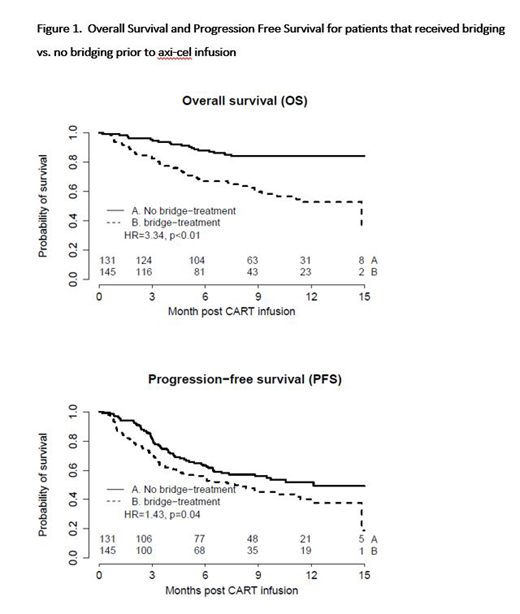
In terms of outcomes, in multivariate analysis correcting for confounding features, there was no statistically significant difference in overall response rate (p = 0.2), complete response rate (p = 0.19), and progression free survival (p = 0.3) between bridging and no bridging groups; but bridging therapy was associated with significantly poorer overall survival (p = 0.001) and lymphoma specific survival (p = 0.019) (figure 1.). Both death due to lymphoma (33.1% vs. 13.0%) and death due to treatment-related mortality (TRM) (6.9% vs. 1.5%) were higher in the bridging group (p <0.001).
Conclusions
Lymphoma patients receiving bridging therapy had poorer prognostic factors at baseline and after axi-cel infusion experienced decreased lymphoma-specific and overall survival compared with patients with no bridging. This inferior outcome raises the possibility that bridging therapy may identify a sub-group of lymphoma patients with a different biology, or alternatively, bridging therapy may have an effect on the host or the tumor microenvironment that may impact CAR-T efficacy. With or without bridging, 7% of patients in our series did not receive axi-cel due to lymphoma progression and/or death. In addition, there may have been patients where bridging prevented progression or death prior to axi-cel infusion. Prospective evaluation of different bridging strategies is warranted to determine if any can improve outcomes after axi-cel, and/or if they should be utilized only for patients requiring emergent disease control during the manufacture period.
Jain:Kite/Gilead: Consultancy. Nastoupil:Bayer: Honoraria; Celgene: Honoraria, Research Funding; Genentech, Inc.: Honoraria, Research Funding; Gilead: Honoraria; Janssen: Honoraria, Research Funding; Novartis: Honoraria; TG Therapeutics: Honoraria, Research Funding; Spectrum: Honoraria. Lin:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; BlueBird Bio: Research Funding; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Sorrento: Membership on an entity's Board of Directors or advisory committees. Lunning:Curis: Research Funding; Janssen Scientific Affairs, LLC: Consultancy, Research Funding; Juno Therapeutics: Consultancy, Research Funding; MiRagen: Research Funding; TG Therapeutics: Consultancy, Research Funding; AbbVie: Consultancy; Bayer: Consultancy; DAVA: Consultancy; Gilead Sciences, Inc.: Consultancy; Kite: Consultancy; Novartis: Consultancy; OncLive: Consultancy; Portola: Consultancy; Seattle Genetics: Consultancy; Spectrum: Consultancy; VANIUM: Consultancy; Verastem: Consultancy. Reagan:Kite, A Gilead Company: Consultancy; Curis: Consultancy; Seattle Genetics: Research Funding. Oluwole:Pfizer: Consultancy; Spectrum: Consultancy; Gilead Sciences: Consultancy; Bayer: Consultancy. McGuirk:Juno Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; Fresenius Biotech: Research Funding; Astellas: Research Funding; Bellicum Pharmaceuticals: Research Funding; Kite Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gamida Cell: Research Funding; Pluristem Ltd: Research Funding; ArticulateScience LLC: Other: Assistance with manuscript preparation. Deol:Novartis: Other: Advisory board; Kite: Other: Advisory board; Agios: Other: Advisory board. Goy:University of Nebraska: Research Funding; Hakensackumc: Research Funding; Astrazenca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Hackensack University Medical Center, RCCA: Employment; Genentech: Other: Grants outside of the submitted work, Research Funding; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work, Research Funding; Pharmacyclics/Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work; Takeda: Other: Grants outside of the submitted work; COTA: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Other: leadership role for profit healthcare company. Hill:Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite: Consultancy, Honoraria; Celegene: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; Takeda: Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria; Genentech: Consultancy, Research Funding; TG therapeutics: Research Funding; Amgen: Research Funding. Munoz:Kyowa: Consultancy, Honoraria, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene/Juno: Consultancy, Research Funding; Genentech: Consultancy, Research Funding, Speakers Bureau; Kite/Gilead: Consultancy, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy; Alexion: Consultancy; Pfizer: Consultancy; Fosunkite: Speakers Bureau; AstraZeneca: Speakers Bureau; Portola: Research Funding; Incyte: Research Funding; Pharmacyclics /Janssen: Consultancy, Research Funding, Speakers Bureau; Bayer: Consultancy, Speakers Bureau; Merck: Consultancy. Chavez:Janssen Pharmaceuticals, Inc.: Speakers Bureau; Kite Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Genentech: Speakers Bureau. Vose:Acerta Pharma: Honoraria, Other: Grants, Research Funding; Bristol-Meyers Squibb Company: Research Funding; Celgene Corporation: Research Funding; Incyte Corporation: Research Funding; Kite Pharma: Honoraria, Other: Grants, Research Funding; Novartis: Research Funding; Seattle Genetics: Research Funding; AbbVie: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Legend Pharmaceuticals: Honoraria. Miklos:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Kite-Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; AlloGene: Membership on an entity's Board of Directors or advisory committees; Precision Bioscience: Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotech: Membership on an entity's Board of Directors or advisory committees; Becton Dickinson: Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees. Neelapu:Cellectis: Research Funding; Allogene: Consultancy; Incyte: Consultancy; BMS: Research Funding; Novartis: Consultancy; Karus: Research Funding; Celgene: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Acerta: Research Funding; Poseida: Research Funding; Unum Therapeutics: Consultancy, Research Funding; Pfizer: Consultancy; Precision Biosciences: Consultancy; Cell Medica: Consultancy. Bennani:Kite Pharma: Other: Advisory board; Kite Pharma: Other: Advisory board; Purdue Pharma: Other: Advisory board; Seattle Genetics: Other: Advisory board; Adicet Bio: Other: Advisory board; Adicet Bio: Other: Advisory board; Adicet Bio: Other: Advisory board; Seattle Genetics: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Kite Pharma: Other: Advisory board; Purdue Pharma: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Purdue Pharma: Other: Advisory board; Seattle Genetics: Other: Advisory board. Andreadis:Pharmacyclics: Research Funding; Novartis: Research Funding; Roche: Equity Ownership; Celgene: Research Funding; Juno: Research Funding; Jazz Pharmaceuticals: Consultancy; Genentech: Consultancy, Employment; Merck: Research Funding; Gilead: Consultancy; Kite: Consultancy. Sehgal:Juno/Celgene: Research Funding; Merck: Research Funding; Kite/Gilead: Research Funding. Locke:Novartis: Other: Scientific Advisor; Kite: Other: Scientific Advisor; Cellular BioMedicine Group Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal