Introduction: Lung cancer (LC) is the second most common cancer in both sexes and is the leading cause of cancer mortality in the United States. Thrombosis is the second leading cause of death in cancer patients and PATP in solid cancer patients were performed in research trials to decrease venous thromboembolism (VTE) rates with ultimate aim to improve survival. We undertook an updated meta- analysis of RCTs to determine the benefit and risk of PATP with low-molecular weight heparins (LMWHs) and direct oral anticoagulants (DOAC) in patients with locally advanced or metastatic LC receiving chemotherapy.
Methods: We performed a comprehensive literature search using MEDLINE and EMBASE databases through June 30, 2019. The references of all potential studies were also reviewed for any additional relevant studies. The RCTs with reduction in VTE as a primary or secondary endpoint and the major bleeding (MB) as a safety outcome were incorporated in the analysis. Mantel-Haenszel (MH) method was used to calculate the estimated pooled risk ratio (RR), and risk difference (RD) with 95% confidence interval (CI). Heterogeneity was assessed with Cochran's Q- statistic. Fixed effects model was applied.
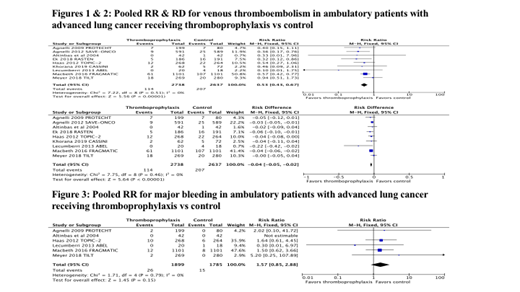
Results: A total of 5,375 patients with LC from five RCTs and a subgroup of another four RCTs were included in our meta-analysis. The prophylactic doses of bemiparin, certoparin, dalteparin, nadroparin, semuloparin and tinzaparin, intermediate dose of enoxaparin and prophylactic dose of rivaroxaban were used in the studies. The duration of LMWH and DOAC ranged from 3 to 6 months. The randomization ratio was 2 to 1 in PROTECHT study and 1 to 1 in all other studies. The I2statistic for heterogeneity was 0, suggesting homogeneity among RCTs. The VTE incidence was 114 (4.16%) in PATP group and 207 (7.85%) in control group with a RR of 0.53 (95% CI: 0.43 to 0.67, P < 0.001). The absolute RD in VTE was -0.04 (95% CI: -0.05 to -0.02, P < 0.001) with an estimate of the number needed to treat (NNT) of 28 to prevent one VTE event. MB events were reported in 26 (1.37%) patients in PATP group compared to 15 (0.84%) in control group according to an analysis of 6 RCTs. The pooled relative risk for MB was statistically non-significant at 1.57 (95% CI: 0.85 to 2.88, P = 0.15).
Conclusions: Our analysis showed that the relative risk reduction is 46% with a NNT of 28 to prevent one VTE without increasing MB events in ambulatory patients with locally advanced or metastatic LC. Selection of appropriate patients who are high risk for VTE is crucial and further studies are required to define high risk subsets of LC patients receiving chemotherapy who may benefit from PATP.
Oo:Janssen and Janssen: Other: Research: site co-investigator ; Medical Education Speakers Network: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal