Introduction
The prevalence of venous thromboembolism (VTE) in patients non-ambulatory from birth has not been well established. Available literature is unclear on the risk of VTE in patients with cerebral palsy (CP), both in the medical and post-surgical settings. Additionally, no large scale, population based studies of VTE rates in patients with severe disabilities have been done. Furthermore, assessing how the risk of VTE evolves with age in this patient population has not been assessed. Therefore, current guidelines regarding VTE prophylaxis do not take a stated position on patients with CP and recommend against thromboprophylaxis in chronically immobilized persons. Our study aims to assess the prevalence of VTE in patients with CP, both ambulatory and non-ambulatory, comparing them to the general population.
Methods
The Explorys platform provides aggregated patient data from many major integrated health systems incorporating tens of millions of patient lives since 1999. Explorys is an IBM database that draws on de-identified data that resides in a cloud-based, HIPAA-enabled, highly secure platform and houses over 63 million unique patients and allows customizable cohort selection as well as longitudinal patient-level data tracking. Patients were sorted based on coded diagnoses of cerebral palsy (CP), wheelchair dependence or wheelchair bound (WD) as a proxy for non-ambulatory status, and venous thrombosis (VTE). A control cohort representing the general population (GP) was generated using patients with a coded diagnosis for any disease excluding those above. Using these codes, we compared VTE prevalence among 4 primary cohorts: (1) general population with neither CP nor WD, (2) patients with CP but without WD, (3) patients with WD but without CP, and (4) patients with both CP and WD. A secondary analysis stratified patients by age decile and compared VTE prevalence between the four cohorts as well as compared rates after removing those with known risk factors for VTE. We also collected distributions of race, gender, and secondary diagnoses to characterize each cohort.
Results
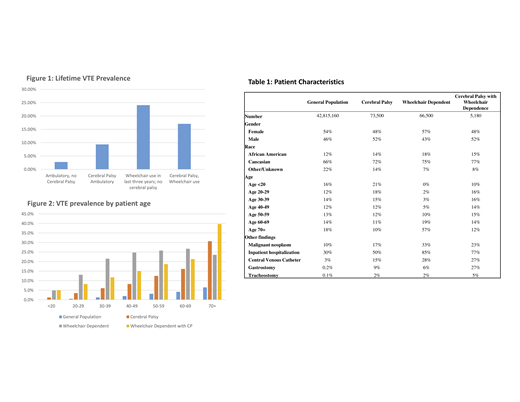
We identified 42,566,360 unique patients, of whom 73,040 had CP but no WD, 65,390 had WD but no CP, and 5100 had both CP and WD (table 1). Among patients without WD, patients with a diagnosis of CP had higher prevalence of VTE than those without (figure 1; 9.36% vs 2.78%, RR 3.37, p <0.0001). Among patients with WD, patients with CP had a lower rate of VTE (17.1% vs 24.1%, RR 0.71, p<0.0001) compared to non-CP patients who were WD. When comparing CP WD to ambulatory non-CP we found that patients with WD and CP had a higher rate of VTE (17.1% vs 2.78%, RR 6.11, p <0.0001). This observed relationship was consistent when stratified by age decile (figure 2), as well as in a secondary analysis of patients with a coded inpatient encounter but no diagnosis for malignancy or central venous catheter insertion with VTE prevalence 4.0% in GP, 4.8% CP no WD, 19.5% WD no CP, and 10.5% CP and WD; all relationships significant at p<0.0001.
Conclusion
This study demonstrates that in non-wheelchair patients, ambulatory patients with CP had a higher rate of VTE compared to those without CP. And although wheelchair bound patients with CP had a higher rate of VTE vs ambulatory non-CP patients, this rate was lower than that observed among patients who are wheelchair dependent for reasons other than cerebral palsy. Our findings suggest that all patients with CP regardless of ambulatory status have a high prevalence of VTE but that in wheelchair bound patients, CP appears to be a protective factor in comparison to those who are wheelchair dependent for reasons other than CP. Both ambulatory and non-ambulatory patients with CP should be considered an at-risk group for VTE when calculating VTE risk scores and should be assessed accordingly when presenting with symptoms suggestive of venous thromboembolism. Finally, our data would suggest that patients who are wheelchair dependent may need to be considered a high-risk group when considering DVT prophylaxis during hospitalization, though further studies are needed to clarify risk in the hospital setting.
Ahuja:XaTexk Inc.: Consultancy, Patents & Royalties, Research Funding; Rainbow Children's Foundation: Research Funding; Bayer: Consultancy; Biovertiv Sanofi: Consultancy; Genentech: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal