Introduction: The safety and efficacy of rurioctocog alfa pegol (BAX 855, SHP-660, TAK-660; Adynovate®; Baxalta US Inc., a Takeda company, Lexington, MA, USA) in patients with severe hemophilia A has been reported previously (Konkle BA et al., Blood 2015, 126:1078-85; Brand B et al., Haemophilia 2016, 22:e251-8; Mullins ES et al., Haemophilia 2017, 23:238-46); however, research describing patient experience with extended half-life (EHL) recombinant factor VIII (FVIII) products outside clinical trials is limited. The objective of this study was to assess real-world utilization of TAK-660 in patients with hemophilia A and describe their clinical profiles before and after switching to TAK-660. Factor consumption and bleed outcomes stratified by age (<18 and ≥18 years) are reported herein.
Methods: This was a retrospective, observational database study of patient data from US specialty pharmacies. Pharmacy data sources included patient records, prescriptions, and patient-reported bleed logs. Informed consent was obtained for all analyzed patient data. Eligible patients with hemophilia A were treated with prophylactic TAK-660 with on-label dosing from November 2015 to September 2018, and had received ≥90 days of FVIII (standard half-life [SHL] or EHL) therapy before switching to TAK-660. Main exclusion criteria were participation in a TAK-660 clinical trial before/during this study, only on-demand treatment before switching to TAK-660, or presence of active FVIII inhibitor requiring treatment and/or use of immune tolerance induction during the study period. Assessments included prior hemophilia therapy, FVIII administration frequency and consumption, and annualized bleeding rate (ABR) before and after switching to TAK-660.
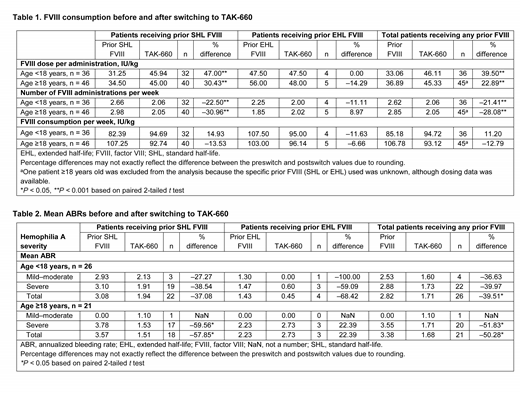
Results: Data was collected from 82 patients (of 61 providers in 44 practices across 25 states in the United States): 44% of the patients (36/82) were <18 years old; 56% (46/82) were ≥18 years old (none were ≥60 years old); 83% (68/82) had severe hemophilia A; and 88% (72/82) had received prior SHL-FVIII treatment. The SHL antihemophilic factor (recombinant) (Advate®; Baxalta US Inc., a Takeda company, Lexington, MA, USA) was used by 67% (55/82) of patients overall, of whom 47% (26/55) were <18 years old and 53% (29/55) were ≥18 years old. Compared with any prior FVIII therapy, switching to TAK-660 increased FVIII dose per administration in patients <18 and ≥18 years old (+39.5% and +22.9%, respectively), while their weekly administration frequency decreased (-21.4% and -28.1%, respectively; Table 1). Weekly FVIII consumption increased in patients aged <18 years (+11.2%) and decreased in those aged ≥18 years (-12.8%). FVIII administration frequency and consumption by prior SHL- or EHL-FVIII are reported in Table 1. ABR data before and after switching were available in 47 of 82 patients. Compared with any prior FVIII therapy, mean ABR decreased in patients aged <18 years (-39.5%; 2.8 to 1.7) and ≥18 years (-50.3%; 3.4 to 1.7) with TAK-660 treatment (Table 2). Changes in mean ABR by prior FVIII therapy and disease severity are reported in Table 2. The small number of patients who received prior EHL FVIII was a limiting factor in the comparison of patients who received prior SHL- and EHL-FVIII therapy.
Conclusions: In patients with hemophilia A previously treated with SHL- or EHL-FVIII products, switching to TAK-660 prophylaxis resulted in a significant decrease in ABR of 40-50% in both age groups analyzed. The adult population (ie, ≥18 years old) showed a tendency for reduced weekly FVIII consumption. These findings from real-world data are in agreement with TAK-660 clinical trial results. The observed differences in FVIII consumption between patients <18 and ≥18 years old may have been in part a result of age-related changes in bleeding patters, growth, and other factors.
Watt:Shire International GmbH, a Takeda company: Employment, Other: a Takeda stock owner. Milligan:Sanofi: Research Funding; Merck: Research Funding; Gilead: Research Funding; Amgen: Research Funding; AbbVie: Research Funding; Trio Health: Employment; Viiv: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal