Background: CAR T cell therapies have demonstrated high response rates in patients (pts) with R/R B cell NHL. Liso-cel is an investigational anti-CD19, defined composition, 4-1BB CAR T cell product administered at target doses of CD4+ and CD8+ CAR T cells. The seamless phase 1 TRANSCEND NHL 001 study evaluated liso-cel in pts with R/R large B cell NHL (NCT02631044). We present data with long-term follow-up from pts treated in the DLBCL cohort.
Methods: Pts aged ≥18 yrs in the DLBCL cohort had R/R DLBCL not otherwise specified (NOS; including transformed from any indolent lymphoma), high-grade B cell lymphoma (HGBCL) with MYC and BCL2 and/or BCL6 rearrangements, primary mediastinal B cell lymphoma (PMBCL), or follicular lymphoma grade 3B (FL3B). Pts had R/R disease after ≥2 lines of therapy and ECOG PS 0‒2. Pts with grade 3/4 cytopenias, mild-moderate organ dysfunction, and secondary CNS lymphoma were eligible. Bridging therapy was allowed, but pts had to have PET-positive disease before lymphodepletion (LD) with fludarabine and cyclophosphamide. Liso-cel was administered at 1 of 3 target dose levels (DLs) of 50×106 (DL1), 100×106 (DL2), or150×106 (DL3) viable CAR+ T cells in the dose-finding cohorts. All dose levels were expanded, and DL2 was chosen as the target DL for dose confirmation. Primary endpoints were treatment-emergent adverse events (TEAEs) and overall response rate (ORR). Secondary endpoints included CR rate, DOR, PFS, PFS ratio, and OS. TEAEs, including investigator-identified neurological events (NE) related to liso-cel, were graded using CTCAE criteria; CRS was graded per the Lee criteria (2014). ORR was assessed by independent review using the Lugano criteria. Data from all liso-cel-treated pts in the DLBCL cohort were analyzed across DLs.
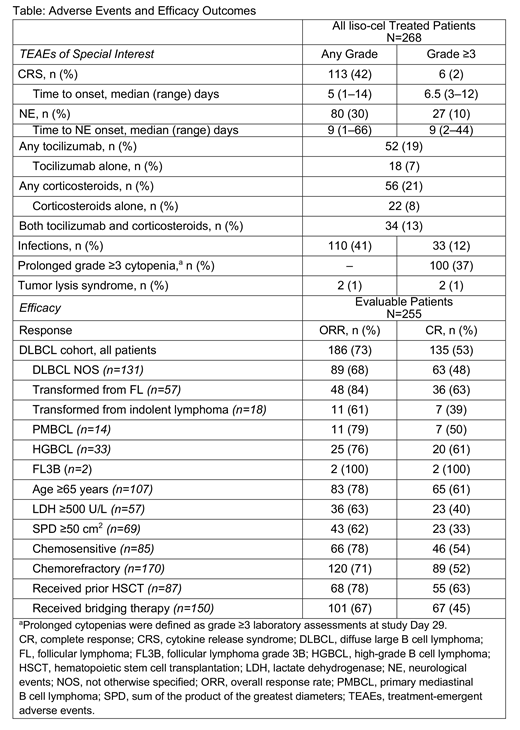
Results: A total of 342 pts were leukapheresed; 268 pts received liso-cel (DL1, n=51; DL2, n=176; DL3, n=41). Twenty-four pts received nonconforming product; product could not be manufactured for 2 pts. Pts did not receive liso-cel primarily due to death (n=33). In the optimized manufacturing process, median time from leukapheresis to liso-cel availability was 24 days. Median age was 63 (range, 18‒86) yrs, including older subpopulations (≥65 yrs, 41%; ≥75 yrs, 10%); 65% of pts were male. At screening, 58% had ECOG PS 1. Pts were heavily pretreated and had aggressive disease: 26% had ≥4 prior lines of systemic therapy (median of 3; range, 1‒8); 34% underwent prior auto-HSCT and 3% prior allo-HSCT; 67% were chemorefractory; and 44% had never achieved CR. 59% required bridging therapy. 22% had LDH ≥500 U/L and 28% had SPD ≥50 cm2 before LD. Outcomes were similar between DLs; therefore, data were pooled. Twenty-five pts were treated in the outpatient setting. Safety analysis showed 79% of pts had grade ≥3 TEAEs, primarily cytopenias (neutropenia, 60%; anemia, 37%; thrombocytopenia, 27%). CRS or NE occurred in 47% of pts. Any grade CRS occurred in 42% of pts at a median onset of 5 days; only 2% had grade ≥3 CRS. NEs occurred in 30% of pts (grade ≥3, 10%) at a median onset of 9 days (Table). 19% of pts received tocilizumab and 21% received corticosteroids for CRS and/or NEs. Four grade 5 TEAEs related to liso-cel and LD occurred (diffuse alveolar damage, pulmonary hemorrhage, multiple organ dysfunction syndrome, cardiomyopathy). Prolonged grade ≥3 cytopenia (based on laboratory assessment at Day 29) was reported in 37% of pts. Safety was similar between age groups, histologies, and pts with organ dysfunction. The study met all primary and secondary efficacy endpoints. Among pts evaluable for efficacy (n=255), ORR was 73% (95% CI, 67‒78); the CR rate was 53% (95% CI, 47‒59). Responses were similar across all pt subgroups (Table). Median DOR was 13.3 mo (95% CI, 8.2‒not reached [NR]) with 10.8 mo of median follow-up; median DOR for pts in CR was NR (13.3‒NR). Median PFS was 6.8 mo (95% CI, 3.3‒11.8). Median OS was 19.9 mo (95% CI, 10.9‒NR). Overall, PFS after liso-cel infusion was substantially longer than PFS from the immediate prior therapy.
Conclusions: Liso-cel demonstrated durable clinical activity with a favorable safety profile in this large study of CAR T cell therapy in R/R large B cell NHL. Low incidence and late onset of CRS and NE allowed for outpatient administration. Clinically meaningful efficacy was observed across histologic subgroups and those with poor prognosis, including pts who were refractory, elderly, comorbid, and/or had high tumor burden.
Abramson:AbbVie Inc, Amgen Inc, Bayer HealthCare Pharmaceuticals, Celgene Corporation, EMD Serono Inc, Genentech, Gilead Sciences Inc, Janssen Biotech Inc, Juno Therapeutics, a Celgene Company, Karyopharm Therapeutics, Kite Pharma Inc, Merck, Novartis, Seattle Gen: Consultancy. Palomba:Kite Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Merck & Co Inc.: Consultancy; MSK (IP for Juno and Seres): Patents & Royalties; Noble Insights: Consultancy; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Seres Therapeutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; STRAXIMM: Membership on an entity's Board of Directors or advisory committees; Evelo: Equity Ownership; Hemedicus: Speakers Bureau. Gordon:Gilead: Other: Advisory Board; Bayer: Other: Advisory Board; Juno/Celgene: Other: Advisory Board, Research Funding; Zylem LLC: Other: co-founder; research in nanoparticles in cancer. Lunning:Spectrum: Consultancy; Seattle Genetics: Consultancy; Portola: Consultancy; OncLive: Consultancy; Novartis: Consultancy; Kite: Consultancy; Gilead Sciences, Inc.: Consultancy; DAVA: Consultancy; Bayer: Consultancy; AbbVie: Consultancy; TG Therapeutics: Consultancy, Research Funding; MiRagen: Research Funding; Juno Therapeutics: Consultancy, Research Funding; Janssen Scientific Affairs, LLC: Consultancy, Research Funding; Curis: Research Funding; VANIUM: Consultancy; Verastem: Consultancy. Wang:Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharmacyclics: Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; MoreHealth: Consultancy, Equity Ownership; Acerta Pharma: Consultancy, Research Funding; Kite Pharma: Consultancy, Research Funding; Guidepoint Global: Consultancy; BioInvent: Consultancy, Research Funding; VelosBio: Research Funding; Loxo Oncology: Research Funding; Celgene: Honoraria, Research Funding; Juno Therapeutics: Research Funding; Aviara: Research Funding; Dava Oncology: Honoraria. Arnason:Celgene/Juno: Consultancy; Regeneron Pharmaceuticals, Inc.: Consultancy. Mehta:Pharmacyclics: Consultancy, Speakers Bureau; Celgene: Consultancy, Speakers Bureau; Spectrum: Consultancy, Speakers Bureau; Imbrium therapeutics: Consultancy; Roche-Genentech: Research Funding; Incyte: Research Funding; Kite/Gilead: Research Funding, Speakers Bureau; Takeda: Research Funding; Rhizen: Research Funding; ADC therapeutics: Research Funding; Forty Seven Inc: Research Funding; Juno/Celgene: Research Funding; Affimed: Research Funding; Seattle Genetics: Research Funding, Speakers Bureau; Astex: Research Funding; TG Therapeutics: Research Funding; miRagen: Research Funding; Kyowa-Kirin: Consultancy, Speakers Bureau; Astra-Zeneca: Speakers Bureau; Sanofi: Consultancy. Maloney:Celgene,Kite Pharma: Honoraria, Research Funding; Juno Therapeutics: Honoraria, Patents & Royalties: patients pending , Research Funding; BioLine RX, Gilead,Genentech,Novartis: Honoraria; A2 Biotherapeutics: Honoraria, Other: Stock options . Andreadis:Juno: Research Funding; Pharmacyclics: Research Funding; Genentech: Consultancy, Employment; Kite: Consultancy; Gilead: Consultancy; Jazz Pharmaceuticals: Consultancy; Roche: Equity Ownership; Novartis: Research Funding; Celgene: Research Funding; Merck: Research Funding. Sehgal:Merck: Research Funding; Juno/Celgene: Research Funding; Kite/Gilead: Research Funding. Ghosh:AstraZeneca: Honoraria, Speakers Bureau; Bristol-Myers Squibb: Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; SGN: Consultancy, Honoraria, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Research Funding; Genentech: Research Funding; Forty Seven Inc: Research Funding. Albertson:Juno Therapeutics, a Celgene Company: Employment, Equity Ownership. Garcia:Celgene: Employment, Equity Ownership. Kostic:Juno Therapeutics, a Celgene Company: Employment. Li:Juno Therapeutics, a Celgene Company: Employment. Kim:Juno Therapeutics, a Celgene Company: Employment. Siddiqi:TG Therapeutics: Research Funding; Kite, A Gilead Company: Research Funding; Seattle Genetics: Speakers Bureau; Janssen: Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; PCYC: Consultancy, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Other: travel support, Research Funding; Celgene: Research Funding; BeiGene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal