Background
The high inter-individual variability in pharmacokinetics (PK) of factor VIII (FVIII) justifies the use of PK-guided prophylaxis, especially in switching between different products. A proposed definition of extended half-life (EHL) FVIII requires improvements of at least 1.3 times in half-life (t1/2) and 1.25 times the area under the curve (AUC) compared to standard half-life (SHL) products (Mahlangu et al. Haemophilia. 2018;24(3):348-358). The objective of this multicenter study was to analyze the results of a PK-guided switch from SHL to EHL in patients with hemophilia A (HA).
Methods
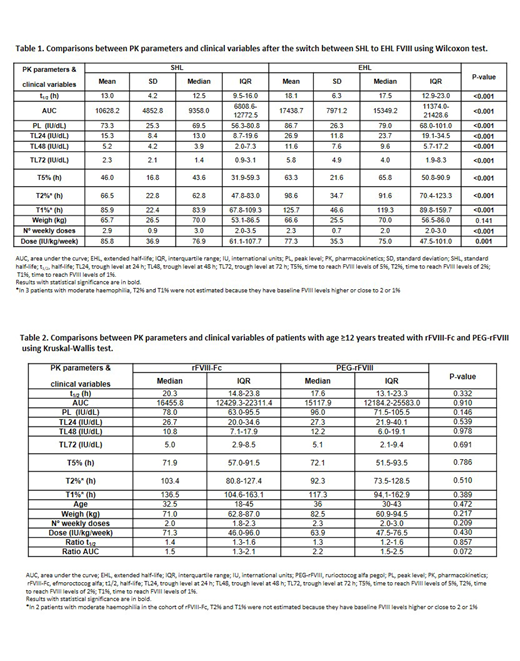
Multicenter comparative, cross-sectional, prospective study in HA severe/moderate patients in prophylaxis analyzing PK differences after switch from SHL to EHL (efmoroctocog alfa [rFVIII-Fc] and rurioctocog alfa pegol [PEG-rFVIII]). WAPPS-Hemo® was used to analyze PK parameters with 2-3 samples: t1/2; AUC, peak level (PL); trough level at 24, 48 and 72 h (TL24, TL48, TL72); and time to reach FVIII levels of 1, 2, 5% (T1%, T2%, T5%). We have also compared the ratio of t1/2 and AUC, the number of weekly doses and the dose/kg/week before and after the switch. Wilcoxon and Kruskal-Wallis tests (SPSS®) were used to compare the PK parameters. Results are expressed with the median and interquartile range (IQR) or range, and mean and standard deviation (SD).
Results
Eighty-one patients from 8 Spanish hospitals were analyzed (61 rFVIII-Fc; 20 PEG-rFVIII), 78 had severe HA and 3 moderate HA. Mean age was 30 years (range=3-64) and no differences in weight were observed between both periods [70 (range=12-116) vs 70 (13.7-116) kg; p=0,141].
Dose/kg/week and weekly infusion frequency were reduced after the switch to EHL, and significant improvements were observed in all PK parameters after the change from SHL to EHL (Table 1). These results were similar in adult and pediatric patients switching to rFVIII-Fc. The median ratios of t1/2 and AUC were 1.3 (IQR:1.2-1.6) and 1.5 (IQR:1.3-2.3) in the entire cohort. These results were reproduced in the subset of patients with ≥12 years treated with rFVIII-Fc and PEG-rFVIII (ratio t1/2: 1.4 [IQR:1.3-1.6]; ratio AUC: 1.6 [IQR:1.3-2.3]), and were slightly in the cohort of 15 patients <12 years treated with rFVIII-Fc (ratio t1/2: 1.3 [IQR:0.9-1.3]; ratio AUC: 1.3 [IQR:1.1-1.9]), the only EHL approved in Europe for children.
After the switch to EHL, weekly dose frequency (median 23.3%, IQR:0-33.3%) and dose/kg/week (median 16%, IQR:5.3-30%) were reduced. In a small subset of 17 patients the dose/kg/week increased a median of 31.4%, and this subset was younger than the patients with dose/kg/week reduction or no changes (median age 18 [IQR:8-22] vs. 33 [IQR:16.5-42.5]). No differences were observed in any of the PK parameters and median ratios of t1/2 and AUC in patients aged ≥12 years treated with rFVIII-Fc vs. PEG-rFVIII (46 rFVIII-Fc; 20 PEG-rFVIII; Table 2).
Conclusions
EHL FVIII have shown significant PK improvements in clinical real practice, allowing to reduce weekly infusion number and dose/kg/week, especially in adult patients. Outside the clinical trial setting, we have observed an increase in t1/2 and AUC ratios accordingly to EHL definition. Comparisons regarding clinical outcomes (bleeding rate after switch) will be performed after a follow-up of 1 year with EHL for the full cohort. WAPPS-Hemo PK-guided switch easily facilitates individualization of prophylaxis with a potential reduction in the cost of treatment and patient perceived outcomes.
Megias:Baxalta US INC.: Research Funding; Grifols: Research Funding. Bonanad Boix:Baxalta US INC.: Research Funding. Berrueco:NovoNordisk: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SOBI: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL-Bering: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Mingot-Castellano:Sobi: Consultancy; Bayer: Consultancy; Amgen: Consultancy; Roche: Consultancy; Novartis: Consultancy; Novonordisk: Consultancy; Takeda: Consultancy; CSL Behring: Consultancy. Cid:Shire, a Takeda company: Honoraria; Novo Nordisk: Honoraria. Sanz:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer-Ingelheim: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Helsinn Healthcare: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen - Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Onconova: Membership on an entity's Board of Directors or advisory committees, Research Funding; Hoffman - La Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal