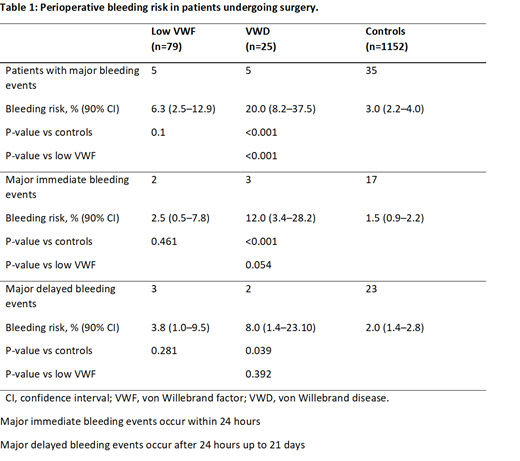
Background: Identifying perioperative bleeding risk in pediatric patients undergoing major surgery is challenging because personal history is often not sufficiently informative and laboratory tests are also more susceptible to pre-analytical variables. Children with low Willebrand add further uncertainty in the treatment options. The aim of this study was to evaluate practices to assess hemorrhagic risk and manage bleeding prophylaxis in children with low von Willebrand factor levels (30 and 50 IU/dL; low VWF), known von Willebrand disease (VWD) or healthy controls undergoing ear, nose and throat (ENT) surgery. Methods: In this retrospective observational study, data from consecutive paediatric patients undergoing ENT surgery between January 1, 2010 and December 31, 2017 at the Turin Paediatric Hospital were analysed. All statistical analyses were performed using STATA (StataCorp. 2013. Been Statistical Software: Release 13. College Station, TX: StataCorp LP). Demographic and clinical characteristics of patients were assessed using the absolute frequencies and percentages for qualitative variables and percentiles for quantitative variables. The risk of bleeding was estimated from the number of patients who experienced bleeding within 21 days as a proportion of the number of patients subjected to surgery. 90% confidence intervals (90% CIs) were estimated for the evaluation of bleeding risk, using the Jeffreys method and any differences in the risk of bleeding between patient groups were tested using a proportions test. To investigate any factors associated with the therapeutic strategy used, multinomial logistic regression models were conducted. Results: Major perioperative bleeding was seen in 6.3% (5/79) of low VWF patients, 3.0% (35/1152) of healthy controls and 20.0% (5/25) of patients with VWD. In low VWF patients, perioperative bleeding prophylaxis was given to 59.5% and included subcutaneously desmopressin (n=21) and VWF-containing concentrate (n=26). Oral or intravenous tranexamic acid was administered to all low VWF patients. Of these patients, one major hemorrhagic event occurred in patients who did not receive prophylaxis and the remaining events occurred in patients treated with VWF-containing concentrate. In patients who received a VWF-containing concentrate, lower VWF ristocetin cofactor levels were observed compared with patients who received desmopressin or were untreated during surgery. No differences in clinical and laboratory features were observed between patients with low VWF treated with desmopressin and those who were untreated. Conclusions: Patients with low VWF have a higher risk of perioperative bleeding compared with healthy controls but a significantly lower risk compared with patients with VWD. The perioperative management of these patients is complex with a high risk of overtreatment. In our experience the caution of keeping the VWF-containing concentrate in the operating room available for use in case of bleeding appears to be a balanced approach. Prospective randomized studies to identify accurate methods of assessing bleeding risk and evaluate the most effective perioperative bleeding prevention are warranted.
Essential bibliography:
Ruchika Sharma and Veronica H. Flood. Advances in the diagnosis and treatment of Von Willebrand disease. Hematology American Society of Hematology Education Program 2017:379-384
Sadler JE. Low von Willebrand factor: sometimes a risk factor and sometimes a disease. Hematology American Society of Hematology Education Program 2009:106-112
Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8(9):2063-2065.
Casey LJ, Tuttle A, Grabell J, et al. Generation and optimization of the self-administered pediatric bleeding questionnaire and its validation as a screening tool for von Willebrand disease. Pediatr Blood Cancer. 2017;64(10)
Mannuccio Mannucci P, Kyrle PA, Schulman S, Di Paola J, Schneppenheim R, Cox Gill J. Prophylactic efficacy and pharmacokinetically guided dosing of a von Willebrand factor/factor VIII concentrate in adults and children with von Willebrand's disease undergoing elective surgery: a pooled and comparative analysis of data from USA and European Union clinical trials. Blood Transfus. 2013;11(4):533-540
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal