Introduction
Emicizumab is a recombinant humanized monoclonal antibody that bridges factor IXa and Factor X and is administered via subcutaneous injection. It provides protection against bleeding in patients with hemophilia A (HA) with and without inhibitors of all ages. Since licensure, the use of emicizumab has increased across hemophilia treatment centers (HTCs). Management of patients on this drug who require procedures has not been well established. The objective of this study was to report peri-procedural hemostasis management as well as bleeding and thrombotic outcomes in a heterogenous group of patients with HA on emicizumab requiring interventional procedures.
Methods
We conducted a multi-center observational study at 3 federally funded Hemophilia Treatment Centers: Children's Hospital of Philadelphia, Virginia Commonwealth University and Children's National Health System. Inclusion criteria included patients with HA initiated on emicizumab prior to May 15th 2019 that underwent a surgical, dental or interventional procedure prior to July 15, 2019. Data extraction included: demographics, diagnosis, inhibitor, emicizumab dosing data, surgery type, factor used during surgery, and bleeding or thrombotic complications during or after surgery.
Results
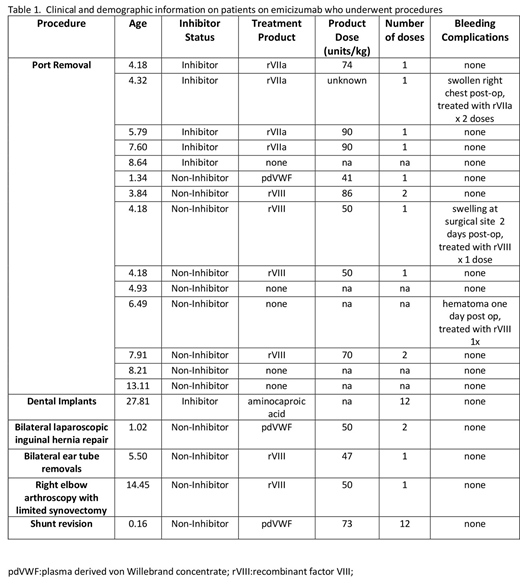
There were a total of 19 procedures in 19 subjects during the study period. 18 were male and all had severe HA. Age at initiation of emicizumab in this cohort ranged from 5 weeks to 27 years; median 5.7 (IQR) yrs. There were 6 patients with an active inhibitor at the time of emicizumab initiation. All patients received emicizumab 3.0 mg/kg weekly x 4 loading doses, followed by either weekly (8), every other week (10), or monthly (1) dosing.
There were 18 minor procedures during the study period (14 port removals and one of each of the following: PE tube removal, laparoscopic inguinal hernia repair, arthroscopy, and dental extraction) and one major procedure (intracranial ventricular shunt revision )(Table 1). Of the 14 port removals, 5 patients (1 with inhibitor) did not receive pre-procedure factor replacement; the other 7 received rVIIa or factor VIII pre-procedure and 2 of these patients received a subsequent dose as part of their plan. Three of the 14 patients that underwent port removal (one with inhibitor) were noted to have swelling and hematoma at the surgical site 1-2 days post-op and received 1-2 doses of subsequent factor to treat these bleeding events; only one of these patients did not receive pre-procedure factor.
The patient who underwent major surgery received multiple doses of FVIII (to keep FVIII levels >50% x 1 week) and did not have bleeding complications.
There were no thrombotic complications.
Conclusions
In general, the 19 patients with HA who required procedures while on emicizumab did well. Port removal was the most common procedure, which was not surprising given the transition from intravenous to subcutaneous prophylaxis. There was variation in practice, particularly in regard to the need for clotting factor prior to port removal. Most patients did well with 0-1 treatments prior to minor procedures, and no additional replacement. Although 3 of the 14 patients required 1-2 unplanned infusions of clotting factor post port removal, there was no clear relationship between pre-procedure factor replacement and post-procedure port hematoma. This observational study provides useful information for providers who manage patients with HA on emicizumab, although larger studies are clearly needed to help determine best practice.
Guelcher:Takeda: Other: Advisory Board; Octapharma: Other: Advisory Board; Genetech: Other: Advisory Board; NovoNordisk: Other: Advisory Board. Butler:Hema-Biologics: Consultancy; pfizer: Other: Advisory board; genetech: Other: Advisory board. Guerrera:Bioverativ: Consultancy; Novo Nordisk: Consultancy; Pfizer: Other: Advisory Board; Bayer: Other: Advisory Board; Shire: Other: Advisory Board; Kendrion: Other: Advisory Board; Genetech: Other: Advisory Board. Raffini:Roche: Other: Advisory Board; CSL Behring: Other: Advisory Board; Bayer: Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal