Host and Tumor Factor XII Drive Ovarian Cancer Maintenance and Progression
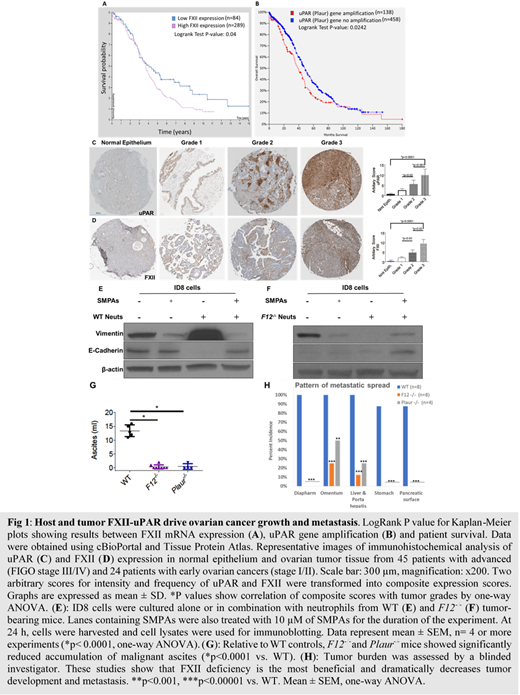
Introduction. Epithelial ovarian cancer (EOC) is the leading cause of cancer death in women.Effective strategies to treat this disease are lacking due to the complexity of pathways involved and the multitude of cells that contribute to EOC biology. To this end, coagulation factor XII (FXII) and its receptor urokinase plasminogen activator receptor (uPAR) represent very promising therapeutic targets because i) uPAR is overexpressed in more than 90% of ovarian cancer patients, ii) FXII has been shown to be upregulated in the peritoneum of EOC patients and promotes EOC dissemination and iii) FXII-uPAR signal and upregulate key neutrophil functions, recently linked to tumor growth and metastasis. Through analysis of the TCGA ovarian cancer patient cohort, we have found that FXII and uPAR are co-expressed in ovarian tumors and their overexpression is associated with decreased overall survival (Fig 1A-B). Given that FXII and uPAR can be produced by both the EOC tumor and the host, the contribution of each of these compartments which has not been examined to date needs to be explored. Thus, we asked if the FXII-uPAR axis in neutrophils and EOC cells synergistically influences tumor biology.
Methods- Results. We utilized a tissue microarray to determine FXII and uPAR expression. Both FXII and uPAR were co-expressed in all major histologic subtypes of EOC tumors but not in normal ovarian epithelium (Fig C-D). We adopted a composite expression score system and found significantly increased expression of FXII and uPAR in high grade tumors compared to low grade (grade 1-2) tumors, independently of tumor subtype and stage (Fig 1C-D). Given prior reports that neutrophils are enriched in the ovarian tumor microenvironment (TME) and our findings that FXII-uPAR are integral to neutrophil activation, tumors were also examined for their neutrophil content. Invariably, as tumor grade advanced, Neutrophil Elastase expression significantly increased (data not shown). We next asked if neutrophils contribute to EOC tumor progression or their recruitment merely correlates with advanced disease. We found that in the presence of neutrophils, EOC cells migrate significantly faster (p<0.0001). Since enhanced tumor cell migration implies a pro-mesenchymal phenotype, we examined if neutrophils facilitate EOC cell epithelial-to-mesenchymal transition (EMT). Murine EOC cancer cells (ID8) were co-cultured with wild type (WT) neutrophils for 24 h; cells were harvested and used for immunoblotting and mRNA studies. We found that WT neutrophils significantly increased the expression of mesenchymal marker(s) vimentin and N-cadherin while they decreased the expression of E-cadherin, suggesting that neutrophils promote EMT of EOC cancer cells (Fig 1E). To investigate whether the FXII-uPAR axis contributed to neutrophil-induced EMT, we next co-cultured ID8 cells with neutrophils from tumor-bearing FXII deleted (F12-/-)mice. In ID8 cells co-cultured with F12-/-neutrophils, EOC induced EMT was blocked. Similarly, treatment with 2 peptide inhibitors that block the FXII-uPAR interaction (collectively termed, SMPAs), reversed the pro-invasive effects of both ID8 cells and WT neutrophils (Fig 1F).
Next, in order to assess whether host FXII could contribute to EOC dissemination and progression we utilized WT, F12-/-, and uPAR deficient (Plaur-/-) mice for in vivo EOC tumor model. After orthotopic injection of ID8 EOC cells, WT mice exhibited faster rates of tumor development and ascitic fluid accumulation (13.4 ± 0.92 ml), relative to F12-/- (0.37 ± 0.26 ml) and Plaur-/- (0.5 ± 0.5 ml) mice (Fig 1G). Blinded examination of tumors showed significantly higher tumor burden in WT mice compared to F12-/-and Plaur-/- mice, with F12-/-mice exhibiting the highest degree of protection (Fig 1H).
Conclusions. Our studies indicate three novel aspects: i) a direct crosstalk between ovarian cancer cells and neutrophils, that enhances tumor cell migration through FXII-uPAR signaling; ii) the presence of neutrophils in the TME induces EMT of cancer cells which is abolished when the FXII-uPAR interaction is inhibited and; iii) abrogation of the FXII-uPAR axis in EOC tumor cells and neutrophils, synergistically restricts the pro-invasive phenotype of ovarian tumor cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal