Introduction: Genetically engineered deletion of ProcR, the gene encoding EPCR, results in early embryonic death, secondary to developmental and functional abnormalities in the placenta (PMID 12218060). ProcR-null embryonic death is prevented by either genetically reducing TF expression or maintaining extraembryonic ProcR expression in placental trophoblast cells (PMID 15956290). These results indicate that placental abnormalities observed in ProcR-null mice are the result of unregulated thrombin generation. Thrombin activates platelets through cleavage of Protease Activated Receptors, Par3 and Par4, present on the surface of murine platelets. The objective of this work was to examine whether decreased responsiveness of maternal platelets to thrombin due to the loss of Par3 would be sufficient to overcome placental abnormality and allow the generation of live ProcR-null mice.
Methods: Par3-/- ProcR+/- mice were generated by breeding Par3-/- and ProcR+/- mice and identified by PCR-based genotyping of tissues obtained by tail biopsies. In experimental crosses, ProcR+/- males were used to sire pregnancies of Par3-/- ProcR+/- females. Reverse genetic crosses, pregnancies of ProcR+/- females sired by Par3-/- ProcR+/- males, served as control. Surgeries were conducted to analyze embryonic survival and placental phenotypes at various stages of gestation. Presence of heart beats and limb movements were used to identify live embryos. We also employed a previously published Meox2Cre-Lox system which selectively ablates ProcR in the embryo while maintaining extraembryonic expression (PMID 15956290). Meox2Cretg/-ProcR-/- mice were generated through this system.
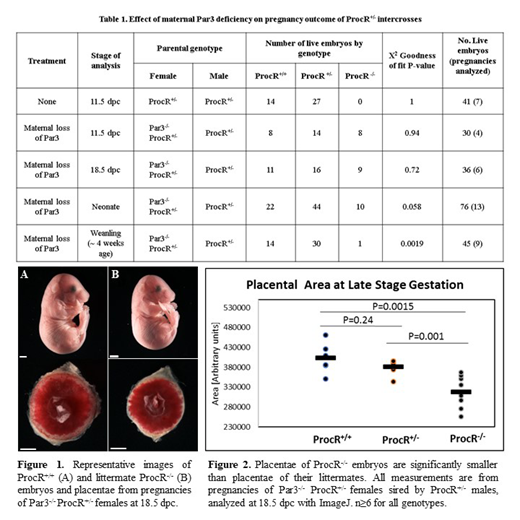
Results: As previously reported, ProcR-/- mice from ProcR+/- intercrosses die on or before 10.5 days post coitum (dpc) (Table 1, Row 1: 25% or 10 ProcR-/- expected, 0.0% observed, 95% CI: 0.0 to 8.6%). ProcR-/- embryos were present in expected Mendelian proportions in pregnancies of Par3-/- ProcR+/- mothers at 11.5 dpc (Table 1, Row 2: 25% or 8 ProcR-/- expected, 26.7% observed, 95% CI: 12.9 to 45.9%) and at 18.5 dpc (Table 1, Row 3: 25% or 9 ProcR-/- expected, 25% observed, 95% CI: 12.1 to 42.2%). Thus, genetic absence of Par3 in the mother completely rescues ProcR-/- mice from intrauterine death. In contrast, in preliminary studies no live ProcR-/- embryos have so far been observed in pregnancies of ProcR+/- females sired by Par3-/- ProcR+/- males at 11.5 dpc (4 ProcR+/+, 5 ProcR+/-, 0 ProcR-/-; 7 abortions; 25% ProcR-/- expected, 0.0% observed, CI: 0.0 to 33.6%), suggesting that paternal loss of Par3 does not confer the same protection. We further noted that ProcR-/- embryos and placentae were significantly smaller than their ProcR+/- and ProcR+/+ littermate controls (Figure 1 and 2).
To our surprise, we found that Par3+/- ProcR-/- progeny of Par3-/- ProcR+/- females were present at reduced frequency as neonates (Table 1, Row 4: 25% or 19 ProcR-/- expected, 13.2% observed, 95% CI: 6.5 to 22.9%) and most did not survive till wean (Table 1, Row 5: 25% or 11 ProcR-/-expected, 2.2% observed, 95% CI: 0.06 to 11.8%). These observations contrast with the published fate of Meox2Cretg/- ProcR-/- mice generated by maintaining ProcR expression on placental trophoblast cells (PMID 15956290) and replicated by us for comparison. As previously reported, we observed 27 Meox2Cre-/- ProcRlox/- and 23 Meox2Cretg/- ProcR-/- at wean (~4 weeks of age) from pregnancies of ProcRlox/lox females sired by Meox2Cretg/- ProcRlox/- males (P= 0.85, χ2 GOF test; 50% expected, 46.0% observed, 95% CI: 31.8 to 60.7%).
Conclusions: Our data demonstrates that the placenta is highly sensitive to local thrombin concentrations and maternal loss of Par3 is sufficient to overcome the obligate requirement of EPCR in the placenta. Second, we show that in stark contrast to ProcR-deficient mice generated through Cre-Lox technology for embryo-specific knockout, germ line deleted ProcR mice rescued through the maternal absence of Par3 die during the neonatal period. These data uncover a critical role of ProcR in postembryonic survival.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal