Background:
Pembrolizumab (PEM), an anti-PD-1 antibody is approved for the treatment of relapsed/refractory cHL based on results of a pivotal trial demonstrating an overall response rate (ORR) of 69% and 22% complete response (CR) rate. To investigate the impact of PEM monotherapy in previously untreated patients (pts), we conducted a phase 2 clinical trial of sequential PEM followed by AVD chemotherapy for untreated cHL. Herein, we report the primary analysis of response (PET2) to single agent PEM in the frontline setting and the results of interim FDG18-FDG PET-CT (PET3) following AVD x 2.
Methods:
Pts > 18 years of age with newly diagnosed cHL stages I-IV, including early stage pts with at least 1 risk factor according to NCCN criteria, were eligible. Pts were treated sequentially with 3 cycles of PEM at 200 mg every 3 weeks followed by an interim PET-CT (PET2) for primary analysis. Subsequently, pts received 4-6 cycles of AVD chemotherapy based on initial stage, with PET-CT's repeated after 2 cycles of AVD and at the end of therapy. There was no consolidative radiotherapy. Response to single agent PEM was assessed by Lugano criteria and decline in total metabolic tumor volume (TMV). We hypothesized that PEM monotherapy prior to AVD would result in a CR rate of 50% and would reduce the frequency of PET-positivity on the interim PET (PET3) following 2 cycles of AVD compared to prior reports.
Results:
Thirty pts enrolled from September 2017- March 2019 with a data cutoff of June 14, 2019. The median age of participants was 30 (range, 21-77), and 4 pts were ages >60. Eleven were male, and the majority (83%) were Caucasian. Twelve pts had early unfavorable disease (6 each with elevated ESR, B-symptoms, and bulky mediastinal masses). Eighteen pts had advanced stage disease (5 Stage 3, 13 Stage 4; IPS score 3 -4 in 7, 1-2 in 10). Adverse risk factors for all 30 pts included bulky disease or large mediastinal mass (n=12), B-symptoms (n=14) and extranodal disease (n=16). Overall therapy was well tolerated and most adverse events were grade 1-2. Related events of any grade included hypertension (n=10), rash (n=9), anemia (n=8), infusion reactions (n=5), elevated liver function tests (n=5), arthralgias (n=4) and hypo- or hyperthyroidism (n=3). Grade 3 and 4 events (whether or not judged to be related) were as follows Gr3: lymphopenia (n=4), febrile neutropenia (n=3), neutropenia (n=3), hypertension (n=2), diarrhea (n=1), hyperglycemia (n=1), and hyponatremia (n=1); Gr 4: neutropenia (n=10); transaminitis (n=1); sepsis (n=1). The only Grade 3 or 4 immune-related adverse event was the one grade 4 transaminitis that resolved with steroid administration and a delay in therapy.
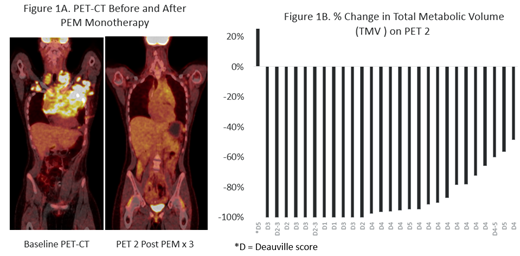
Eleven of 30 pts achieved CR (37%; Deauville 1-3) following initial PEM monotherapy including three with large mediastinal masses. Four additional patients with bulky disease and four others had major reductions in disease following PEM monotherapy, but did not achieve CR by Lugano criteria (Fig 1A). To better characterize the depth of response, we quantitated the decline in total metabolic tumor volume (TMV) (Fig 1B). Eight of the 17 pts with < CR to PEM monotherapy for whom TMV could be analyzed had > 90% reduction in TMV. A single pt had an atypical response with clearance of original disease sites, but development of new sites that resolved after two cycles of AVD. Across all pts, the CR (Deauville 1-3) rate following 2 cycles of AVD, was 100%. Median follow up is 6.5 (range: 1 - 12) months among those who have completed therapy. No pt has required a change in treatment, relapsed, or progressed, with progression-free and overall survival rates both at 100%.
Conclusion:
Although the study did not meet its primary endpoint, our data demonstrate that 3 cycles of PEM is highly active in pts with newly diagnosed early unfavorable or advanced stage cHL. PEM monotherapy resulted in > 90% reduction in TMV in the majority of pts, and when followed by AVD x's 2, CR in 100%. No pts have experienced progression or change of therapy to date and treatment was overall very well tolerated. This radiotherapy- and bleomycin-free approach warrants further investigation in larger trials to confirm response rates and assess efficacy compared with other novel combinations in the frontline setting.
Pro:Takeda: Consultancy, Honoraria, Other: Travel Expenses; Celgene: Consultancy, Honoraria; Kyowa Hakka Kirin: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria, Other: Travel Expenses, Research Funding. Karmali:Gilead/Kite; Juno/Celgene: Consultancy, Speakers Bureau; Takeda, BMS: Other: Research Funding to Institution; Astrazeneca: Speakers Bureau. Gordon:Gilead: Other: Advisory Board; Juno/Celgene: Other: Advisory Board, Research Funding; Zylem LLC: Other: co-founder; research in nanoparticles in cancer; Bayer: Other: Advisory Board. Winter:Merck: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal