Introduction
Terminally differentiated effector memory (TEMRA) cells have been recent revealed as sub-population of CD8 T cells, and they have been expected to act like memory-like properties in peripheral blood. However, the precise characteristics or function of these cells have not been defined. Particularly, these cells have been found even in cancer tissues including tumor-infiltrating effector CD8+ T cells (TILEM). The study investigated the phenotypic or genomic difference of CD8+ TEMRA cells which extracted from not only peripheral blood of healthy and cancer patients but also tumor tissues of cancer patients.
Material and methods
Peripheral blood or tumor tissues from normal donors and patients with lung cancer or Hodgkin lymphoma were collected. Three different sources of CD8+ TEMRA cells, which from healthy peripheral blood (hTEMRA), patient peripheral blood (pTEMRA) and TIL (TIL TEMRA), were obtained and then analyzed for phenotypic and genomic differences. CD8+ T cells were isolated using magnetic-activated cell sorting (MACS), and sub-type cells were isolated using FACS (fluorescence-activated cell sorting) based on phenotypic factors (TEMRA; CD8+ CCR7- CD45RA+). Tumor tissues from patients with lung cancer were digested with enzyme and preceded to the same manner as peripheral blood. Next-generation sequencing (NGS) was used to characterize the TEMRA by using Hiseq2000 (Illumina) on cDNA synthesized from RNA extracted from sorted cells (TEM; CD8+ CCR7- CD45RA-, TEMRA; CD8+ CCR7- CD45RA+). All experiments were performed after obtaining the informed consent approved by the institutional ethical committee (IRB).
Results
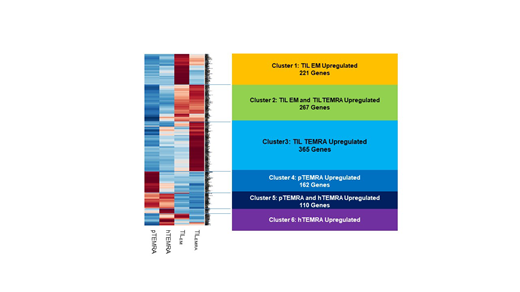
CD8+ TEMRA exist with similar proportion in cancer tissues as well as peripheral blood in lung cancer patients. TIL TEMRA was distinguished from regulatory T cells without expression of CD25 and foxp3. All healthy or patient TEMRA cells including TIL TEMRA showed similar expression level of exhaustion phenotypes (CTLA4, PD-1 and Tim3) (p>0.05). However, TIL TEMRA cells exhibited significantly less amounts of T cell exhaustion markers (PD-1 and Tim3) than those of TILEM cells. Genomic background of blood-derived TEMRA differs from tumor tissue-derived TEMRA based on NGS assessment. Moreover, unlike general CD8+ TILEM, which consist mostly of exhausted effector cells due to tumor-derived immunosuppression, CD8+ TIL TEMRA showed the different genomic background. The transcriptional factors (T-bet and Eomes) or genomic patterns of CD8+ TIL TEMRA represented less exhausted, proliferative and cytotoxic than CD8+ TILEM. However, CD8+ TIL TEMRA might be induced into secondary effector cells after TCR re-stimulation.
Conclusion
Genomic and functional characteristics of CD8+ TIL TEMRA distinct from not only TIL TEM but also peripheral TEMRA.in cancer patients. This research could define the different subset of CD8+ TILs in cancer and give a clue for different response or modulating therapeutic response of immune checkpoint inhibitor by using CD8+ TIL TEMRA cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal