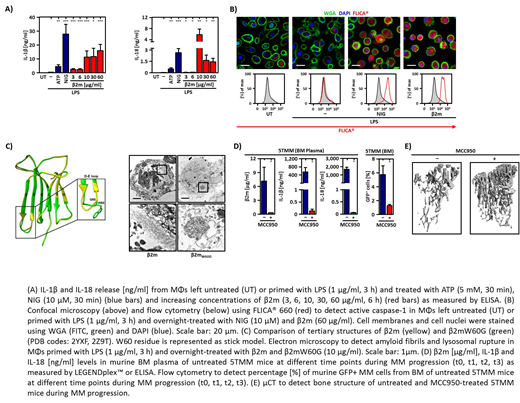
Multiple myeloma (MM) is closely dependent on cross-talk between malignant plasma cells and cellular components of the inflammatory bone marrow milieu, which promotes disease progression, bone destruction and immune-impairment. However, the molecular mechanisms initiating this inflammatory microenvironment remain poorly defined. Beta-2-microglobulin (β2M) accumulates during MM and represents an established prognostic biomarker, but little is known regarding its immunoregulatory potential. Here, we found that β2m triggers an enhanced caspase-1 dependent release of mature IL-1β (15.8 ± 4.5 ng/ml) and IL-18 (5.8 ± 2.1 ng/ml) by macrophages in a NLRP3 dependent way. Activation of the NLRP3 inflammasome was initiated, after phagocytosis of β2m and subsequent lysosomal rupture due to amyloid fibril formation in lysosomes, in a process which partially involved lysosomal protease cathepsin B. β2m mediated NLRP3 inflammasome activation also occurs in tumor-associated macrophages (TAMs) of MM patients leading to the release of IL-1β and IL-18 towards MM cells. Moreover, TAMs in a murine MM model (5TMM) displayed an active NLRP3 inflammasome as well. Treatment of 5TMM mice with MCC950, a selective NLRP3 inhibitor, reduced tumor progression and thus attenuated the severity of MM. Our findings shed new light on β2m as macrophagic NLRP3 inflammasome activator which contributes to MM cell progression, and open a potential novel avenue for therapies of this severe malignancy.
Bruns:Morphosys AG: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal