Sickle cell disease (SCD) is an inherited hemoglobinopathy that frequently causes neurological complications, such as stroke, silent cerebral infarct (SCI) and other forms of brain injury, including loss of cognition. SCI is defined as any ischemic lesion visible on T2-weighted magnetic resonance imaging (MRI) that is not associated with a focal neurologic deficit in the same vascular distribution, and is the most commonly recognized form of brain injury in SCD. SCI is associated with decreased neurocognitive function and increased risk for new or enlarging SCI/stroke. The purpose of this study was to use mass spectrometry (MS) based proteomics to discover and validate plasma brain proteins associated with brain injury in children with SCD.
The plasma samples used for the proteomics discovery analyses were from two groups: screening samples of participants in the Silent Cerebral Infarct Multi-Center Clinical Trial (SIT Trial) (n=15), and 6 age-matched, healthy non-SCD controls, three of whom had sickle cell trait. Samples from children with SCD were divided into two groups: those with SCI (n=7) and those without SCI (n=8) matched for age, hemoglobin (Hb) and white blood cell counts. To identify circulating markers of SCI, plasma proteomes were analyzed using a sequential separation approach of Hb and top abundant plasma protein depletion, followed by reverse phase separation of intact proteins, trypsin digestion and tandem MS. Neurogranin (NRGN), a small calmodulin-binding synaptic protein (PMID 9886843), was the most abundant brain-enriched protein found in the plasma of children with SCD. We developed a NRGN sandwich immunoassay for verification of MS analysis results in an independent cohort from the SIT Trial longitudinal samples. For this verification study, plasma samples from the SIT Trial and 25 normal controls were tested for NRGN levels at study entry and exit by immunoassay. The majority of the SCD samples were obtained from an ancillary study to collect longitudinal samples that started after the SIT Trial had begun. There was no overlap between the discovery and the verification group samples. The normal control samples were from 25 healthy, age and gender comparable non-SCD pediatric controls unrelated to the SIT Trial, without evidence of acute/chronic illness.
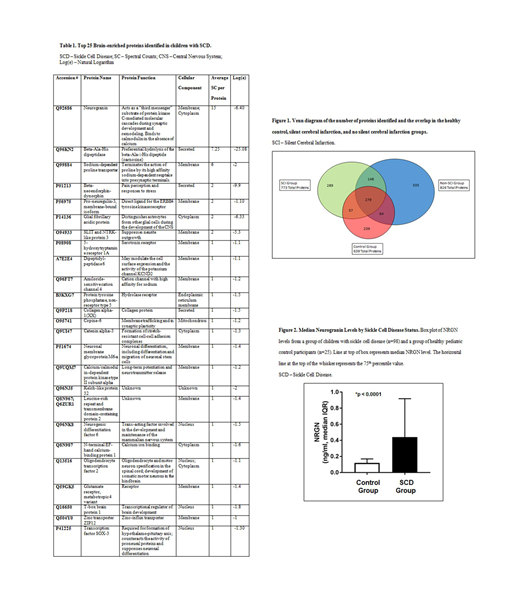
From the discovery SCD group, 1172 unambiguous proteins were identified; 639 proteins were identified from normal controls, as shown in Figure 1. Twenty-five percent (289/1172) of these proteins were found solely in the SCI group. Our MS protein identification data were filtered against this list of expressed brain proteins to produce a composite list of brain proteins found in plasma from children with SCD, but not found in plasma from age, gender and race-matched healthy control children. Twenty-five plasma proteins known to be expressed in one or more cell type enriched in the human brain based on bioinformatics searches were more abundant in the SCD vs control groups, as shown in Table 1.
Median NRGN levels were higher at study entry in children with SCD (0.44 ng/mL, N=98), in comparison to a healthy, non-SCD control group (0.12 ng/mL, N=25, p < 0.0001), as shown in Figure 2. Using the longitudinal samples from the SIT Trial, there were no differences in NRGN levels between the observation group and the treatment group (p=0.35) over time. There was no significant difference in median NRGN levels between the observation group and the treatment group at the initial visit (0.36 vs 0.19, p=0.069) or final visit (0.39 vs 0.52, p=0.52). Using the initial visit samples from the SIT Trial, there was no significant difference in median NRGN levels between the non-SCI (n=35) and SCI groups (n=61) (p=0.073). There was no significant correlation between NRGN levels and neuropsychological measures of executive function (rho=0.089, p=0.58) and IQ (rho=-0.081, p=0.62).
Our non-biased proteomic discovery studies demonstrate that NRGN levels are increased in children with SCD, when compared to normal children. Additional studies will be necessary to determine whether NRGN levels correlate with specific forms of neurologic injury. Further validation studies of larger cohorts with a multi-analyte ELISA of these brain proteins identified in plasma (Table 1) are warranted, and could help establish possible links between brain-specific proteins and neurocognitive outcomes.
Lance:NIH: Research Funding; KKI: Research Funding. Faulcon:FDA: Employment, Other: Although the author is a FDA/CTP employee, this work was not done as part his/her official duties. This publication reflects the views of the author and should not be construed to reflect the FDA/CTP's views or policies.. Fu:GSK: Employment. Yang:ImmunArray: Patents & Royalties: ImmunArray. Strouse:Global Blood Therapeutics: Consultancy. Barron-Casella:Mast Pharmaceudicals - spouse: Consultancy; ImmunArray - spouse: Patents & Royalties. Van Eck:ImmunArray: Consultancy, Patents & Royalties. Casella:NIH: Research Funding; Mast Pharmaceuticals: Consultancy; Immunoarray Ltd.: Patents & Royalties. Everett:ImmunArray: Consultancy, Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal